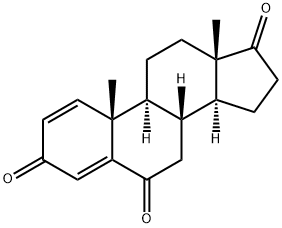
Exemestane
Synonym(s):6-Methyleneandrosta-1,4-diene-3,17-dione;Exemestane
- CAS NO.:107868-30-4
- Empirical Formula: C20H24O2
- Molecular Weight: 296.4
- MDL number: MFCD00866994
- EINECS: 643-090-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-02 11:37:33
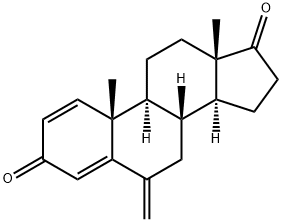
What is Exemestane?
Absorption
42%
Toxicity
Convulsions
Description
Exemestane was launched in US and other countries for the treatment of estrogendependent
tumors and postmenopausal breast cancer. It is a novel steroidal compound
structurally related to the natural substrate for the biosynthesis of estrogen,
androstanedione, and can be synthesized by methylidenation of androsta-1, 4-dien-17-beta-ol-3-one in 6 position then oxidation of the alcohol function.
Exemestane is an
irreversible inactivator of the aromatase enzyme system, so inducing in vivo a dose-related
sustained suppression of serum estrogen and minimal endocrine activity. It is the first
steroidal representative of the third-generation of orally active aromatase inhibitors with a
highly potent and selective mechanism of action, displaying good tolerability and safety
profile.
Chemical properties
white to light yellow crystal powder
Originator
Farmitalia Carlo Erba (Italy)
The Uses of Exemestane
Labeled Exemestane, intended for use as an internal standard for the quantification of Exemestane by GC- or LC-mass spectrometry.
The Uses of Exemestane
An antineoplastic (hormonal)
The Uses of Exemestane
antiinfective
Background
Exemestane is an oral steroidal aromatase inhibitor used in the adjuvant treatment of hormonally-responsive (also called hormone-receptor-positive, estrogen-responsive) breast cancer in postmenopausal women. It irreversibly binds to the active site of the enzyme resulting in permanent inhibition.
Indications
For the treatment of advanced breast cancer in postmenopausal women whose disease has progressed following tamoxifen therapy.
Definition
ChEBI: A 17-oxo steroid that is androsta-1,4-diene-3,17-dione in which the hydrogens at position 6 are replaced by a double bond to a methylene group. A selective inhibitor of the aromatase (oestrogen synthase) system, it is used in the treatment of advanced brea t cancer.
Manufacturing Process
0.50 g of 6-methylenandrost-4-ene-3,17-dione and 0.57 g of dichlorodicyanobenzoquinone were refluxed in 20 ml of anhydrous dioxane for about 15 hours. To remove the DDQ the suspension was filtered through alumina. After evaporation of the solvent the residue was dissolved in ethyl acetate, the organic layer washed with water, dried over sodium sulfate and the solvent removed under vacuum. The crude product was chromatographed on silica gel using hexane/ethyl acetate to yield 0.25 g of pure 6- methylenandrosta-1,4-diene-3,17-dione, m.p. 188-191°C, λmax 247 nm (ε 13.750).
brand name
Aromasin (Pharmacia& Upjohn).
Therapeutic Function
Antineoplastic
General Description
Exemestane, 6-methylenandrosta-1,4-diene-3,17-dione (Aromasin), is the first steroid-basedaromatase inhibitor approved for the treatment of breastcancer in the United States. It is a mechanism-based inactivatorthat irreversibly inhibits the enzyme. Plasma estrogenlevels are reduced by 85% to 95% within 2 to 3 days, and effectslast 4 to 5 days. Exemestane does not inhibit any of themajor cytochromes P450 and has essentially no interactionwith steroid receptors, with only a very weak affinity for theAR. The 17β-hydroxyexemestane reduction product, however,has much higher affinity for the AR than the parent(still several fold less than DHT, 0.28% for parent vs. 30%for metabolite). The clinical significance of the affinity islikely minimal because of the low levels of the metaboliteproduced.
Hazard
A reproductive hazard.
Biochem/physiol Actions
Exemestane is a steroidal antiestrogen and irreversible aromatase inhibitor. Exemestane acts as a false substrate for the aromatase enzyme. Exemestane also prevents the conversion of androgens to estrogens and is used to treat estrogen-dependent breast cancer.
Pharmacokinetics
Aromatase is an enzyme that converts hormones to estrogen in the body's adrenal glands. The aromatase inhibitors (AIs) are drugs that reduce estrogen levels by blocking the action of aromatase in the adrenal glands. The selective AIs (SAIs) selectively reduce levels of estrogen without interfering with levels of other steroid hormones that are produced by the adrenal gland. Drugs in this class include anastrozole (Arimidex ?), letrozole (Femara ?) and exemestane (Aromasin ?).
Clinical Use
Irreversible, steroidal aromatase inhibitor:Treatment of breast cancer
Metabolism
Metabolised via oxidation by the cytochrome P450 isoenzyme CYP3A4, and via reduction by aldoketoreductase. Metabolites are excreted in the urine (39-45%) and faeces (36-48%).
Side Effects
Hot flashes, hair loss, bone pain, tiredness, unusual sweating, nausea, diarrhea, dizziness, and trouble sleeping may occur during taking Exemestane.
References
[1] franco buzzetti, enrico di salle, antonio longo, gabriella briatico. synthesis and aromatase inhibition by potential metabolites of exemestane (6-methylenandrosta-1,4-diene-3,17-dione). steroids. november 1993. 58(11): 527-532.
[2] gustavo de albuquerque cavalcanti, bruno carius garrido, felipe dias leal, monica costa padilha, xavier de la torre, francisco radler de aquino neto. detection of new urinary exemestane metabolites by gas chromatography coupled to mass spectrometry. steroids. september–october 2011. 76(10-11): 1010-1015.
[3] stephanie a. jones, stephen e. jones. exemestane: a novel aromatase inactivator for breast cancer. clinical breast cancer. october 2000. 1(3): 211-216.
Properties of Exemestane
| Melting point: | 155.13°C |
| Boiling point: | 453.7±45.0 °C(Predicted) |
| Density | 1.13±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥20mg/mL |
| form | powder |
| color | white to off-white |
| optical activity | [α]/D +250 to +300°, c = 1 in methanol |
| InChI | InChI=1S/C20H24O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11,14-16H,1,4-5,7,9-10H2,2-3H3/t14-,15-,16-,19+,20-/m0/s1 |
| CAS DataBase Reference | 107868-30-4(CAS DataBase Reference) |
Safety information for Exemestane
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H360:Reproductive toxicity H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Exemestane
| InChIKey | BFYIZQONLCFLEV-DAELLWKTSA-N |
| SMILES | C1(=O)C=C2[C@](C)(C=C1)[C@]1([H])[C@]([H])([C@@]3([H])[C@@](CC1)(C)C(=O)CC3)CC2=C |
Exemestane manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Exemestane 98%View Details
Exemestane 98%View Details -
 107868-30-4 98%View Details
107868-30-4 98%View Details
107868-30-4 -
 Exemestane 98%View Details
Exemestane 98%View Details
107868-30-4 -
 107868-30-4 99%View Details
107868-30-4 99%View Details
107868-30-4 -
 Exemestane 98%View Details
Exemestane 98%View Details
107868-30-4 -
 Exemestane 107868-30-4 98%View Details
Exemestane 107868-30-4 98%View Details
107868-30-4 -
 107868-30-4 Exemestane 99%View Details
107868-30-4 Exemestane 99%View Details
107868-30-4 -
 Exemestane CAS 107868-30-4View Details
Exemestane CAS 107868-30-4View Details
107868-30-4
