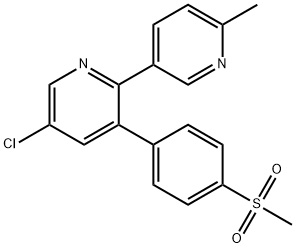
Etoricoxib
Synonym(s):5-Chloro-3-[4-(methylsulfonyl)phenyl]-2-(2-methyl-5-pyridinyl)pyridine;5-Chloro-6′-methyl-3-[4-(methylsulfonyl)phenyl]-2,3′-bipyridine
- CAS NO.:202409-33-4
- Empirical Formula: C18H15ClN2O2S
- Molecular Weight: 358.84
- MDL number: MFCD06797512
- EINECS: 682-421-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-20 21:15:22
What is Etoricoxib?
Absorption
Bioavailability is 100% following oral administration.
Toxicity
This reduced activity is the cause of reduced gastrointestinal toxicity, as demonstrated in several large clinical trials performed with different COXIB (see below links on NEJM and The Lancet). Some clinical trials and meta-analysis showed that treatment with COXIB lead to increased incidence of cardiovascular adverse events compared to placebo
Description
Etoricoxib is a COX-2 inhibitor developed as a follow-up of rofecoxib for treating osteoarthritis, rheumatoid arthritis, dysmenorrhoea, gout, ankylosing spondylitis and pain. Several processes describe the preparation of etoricoxib in 4 or 5 steps from 6- methyl nicotinate. The critical step is the novel pyridine construction using the annulation of a ketosutfone with a vinamidinium synthon. In human whole blood, in vitro, the IC50 value obtained for inhibition of COX-2 is 1.1 μM compared to 116 μM obtained for inhibition of COX-1. Thus, etoricoxib is the most selective COX-2 inhibitor to date, with a COX-IKOX- 2 ratio of IC50 values of 106 for etoricoxib as compared to 35, 30, 7.6 for rofecoxib, valdecoxib and celecoxib, respectively. Its in vivo potency is generally comparable to that of rofecoxib in animal models against inflammation (carrageenan-induced paw edema), pyrexia (LPS-induced pyresis), pain (carrageenan-induced hyperalgesia) and arthritis (adjuvant-induced arthritis).
Chemical properties
Off-White Powder
Originator
Merck & Co (USA)
The Uses of Etoricoxib
The active substance etoricoxib belongs to a group of medicines called selective COX-2 inhibitors. These belong to a family of medicines called non-steroidal anti-inflammatory drugs (NSAIDs). Etoricoxib helps to reduce the pain and swelling (inflammation) in the joints and muscles of people 16 years of age and older with osteoarthritis, rheumatoid arthritis, ankylosing spondylitis and gout. It is also used for the short-term treatment of moderate pain after dental surgery in people 16 and older. Labeled Etoricoxib, intended for use as an internal standard for quantifying Etoricoxib by GC- or LC-mass spectrometry.
The Uses of Etoricoxib
A specific inhibitor of COX-2 .
The Uses of Etoricoxib
anti-inflammatory, analgesic;cyclooxygenase inhibitor
The Uses of Etoricoxib
For the treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, chronic low back pain, acute pain and gout.
The Uses of Etoricoxib
Etoricoxib is a dipyridinyl compound that demonstrates high in vitro and ex vivo selectivity for COX-2 over COX-1 in several assays, e.g., in the production of PGE2 by CHO cells expressing either COX-2 (IC50 = 79 nM) or COX-1 (IC50 > 50 μM). Oral etoricoxib is well absorbed and metabolized extensively via oxidation, with metabolites excreted largely in the urine.[Cayman Chemical]
Indications
For the treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, chronic low back pain, acute pain and gout.
Background
Etoricoxib is a new COX-2 selective inhibitor. Current therapeutic indications are: treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, chronic low back pain, acute pain and gout. Like any other COX-2 selective inhibitor, Etoricoxib selectively inhibits isoform 2 of cyclo-oxigenase enzyme (COX-2) to reduce the generation of prostaglandins (PGs) from arachidonic acid. It is approved in more than 60 countries worldwide but not in the US.
What are the applications of Application
Etoricoxib is a specific inhibitor of COX-2
Definition
ChEBI: A member of the class of bipyridines that is 2,3'-bipyridine which is substituted at the 3, 5, and 6' positions by 4-(methylsulfonyl)phenyl, chlorine, and methyl groups, respectively.
brand name
Arcoxia
Pharmacokinetics
Etoricoxib is well tolerated with dose-proportional pharmacokinetics. It does not affect on bleeding time or platelet ag regation. The gastrointestinal tolerability of etoricoxib is excellent, as demonstrated by [51Cr] excretion models in rats and squirrel monkeys. Moreover, etoricoxib, unlike naproxen, is not associated with significant inhibition of gastric mucosal PGE2 synthesis compared to placebo. Etoricoxib is highly absorbed, with a tmax of 1.5 h and a half-life of approximately 15-22h. Five metabolites, weak inhibitors of COX-1 and COX-2, have been identified after renal excretion. Finally, although multiple CYP enzymes are involved in the metabolism of etoricoxib (CYP3A4 being the major contributor), etoricoxib is not a potent CYP3A4 inhibitor or inducer. In patients undergoing molar extraction, etoricoxib showed similar efficacy to naproxen sodium with a longer duration of analgesia than acetaminophen/codeine (approximately >24 h, 22 h and 5.2 h, respectively) and a better total pain relief score over 8 h. Similar efficacy of etoricoxib and naproxen was also seen in patients suffering from osteoarthritis. In treating rheumatoid arthritis and ankylosing spondylitis, etoricoxib demonstrated significantly superior efficacy to naproxen and placebo. Etoricoxib did not affect the pharmacokinetics of prednisolone (i.v. or p.0.), and its co-administration with antacids showed insignificant effects on maximal concentration and absorption.
Pharmacokinetics
Etoricoxib is rapidly absorbed, with an oral bioavailability of 80 to 100%, and reaches maximum plasma concentrations in 1 to 2 hours after dosing. Food decreases the rate of absorption but has no effect on the extent of absorption. It exhibits a long elimination half-life of approximately 22 hours, demonstrating linear plasma pharmacokinetics with no accumulation during multiple dosing.
Clinical Use
Etoricoxib is a selective COX-2 inhibitor being developed for postsurgical treatment of dental pain (120 mg) and osteoarthritis. It has a methylsulfonyl group common to the other coxib inhibitors.
Synthesis
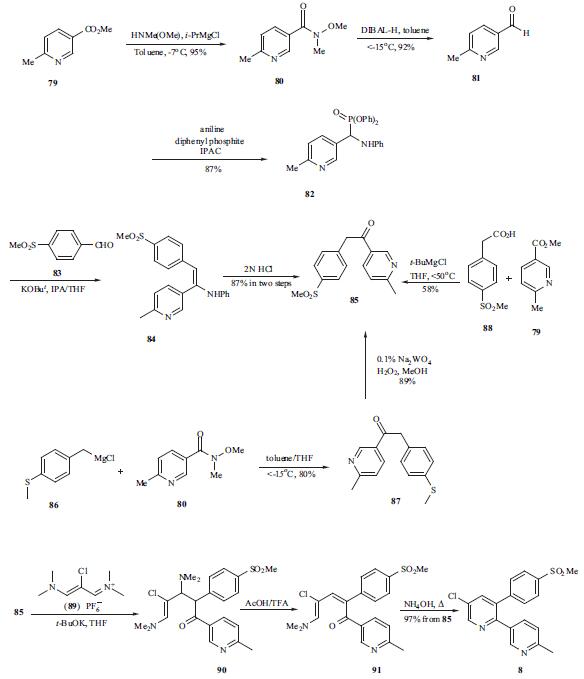
The synthesis of etoricoxib (8) was explored extensively by the Merck process research group. Key intermediate 85 was synthesized through at least three different routes. In the Horner-Wittig approach, 6-methyl methylnicotinate (79) was converted into Weinreb amide 80 in 95% yield. Amide 80 was then converted to aldehyde 81 via a DIBAL-H mediated reduction. Subsequent treatment of a solution of aldehyde 81 in isopropyl acetate with aniline and diphenyl phosphite provided N,P-acetal 82 in 87% yield. The Horner-Wittig reaction of N,P-acetal 82 with 4-methanesulfonylbenzaldehyde (83) furnished enamine 84, which was hydrolyzed to ketosulfone 85. A Grignard approach was also developed in the preparation of ketosulfone 85. Addition of Grignard reagent 86 to Weinreb amide 80 in toluene/THF provided ketosulfide 85 in 80% yield. Tungstate-catalyzed oxidation of ketosulfide 87 using hydrogen peroxide provided ketosulfone 85 in 89% yield by simple filtration. Ketosulfone 85 was prepared through Claisen condensation protocol as well. Thus, reaction of 4-methanesulfonyl phenyl acetic acid (88) with methyl nicotinate 79 under Ivanoff condition, i.e., the magnesium dianion in THF, resulted 58% yield of ketosulfone 85. Treatment of ketosulfone 85 with a three-carbon electrophile, 2-chloro-N,Ndimethylaminotrimethinium hexafluorophos-phate (89) in the presence of potassium t-butoxide at ambient temperature resulted adduct 90. Inverse quench of adduct 90 into a mixture of HOAc /TFA led to the putative intermediate 91. Ring closure of the pyridine ring occurred upon heating at reflux in the presence of an excess of aqueous ammonium hydroxide to give desired etoricoxib (8) in 97% yield in a one-pot process from 85.
Drug interactions
Potentially hazardous interactions with other drugs ACE inhibitors and angiotensin-II antagonists: antagonism of hypotensive effect; increased risk of nephrotoxicity and hyperkalaemia. Analgesics: avoid concomitant use of 2 or more NSAIDs, including aspirin (increased side effects); avoid with ketorolac, increased risk of side effects and haemorrhage. Antibacterials: possibly increased risk of convulsions with quinolones; concentration reduced by rifampicin. Anticoagulants: effects of coumarins and phenindione enhanced; possibly increased risk of bleeding with heparin, dabigatran and edoxaban - avoid long term use with edoxaban. Antidepressants: increased risk of bleeding with SSRIs and venlaflaxine. Antidiabetic agents: effects of sulphonylureas enhanced. Antiepileptics: possibly increased phenytoin concentration. Antivirals: increased risk of haematological toxicity with zidovudine; concentration possibly increased by ritonavir. Ciclosporin: may potentiate nephrotoxicity Cytotoxics: reduced excretion of methotrexate; possibly reduced excretion of pemetrexed; increased risk of bleeding with erlotinib. Diuretics: increased risk of nephrotoxicity; antagonism of diuretic effect; hyperkalaemia with potassium-sparing diuretics. Lithium: excretion decreased. Pentoxifylline: increased risk of bleeding. Tacrolimus: increased risk of nephrotoxicity.
Metabolism
Hepatic, primarily via CYP3A4.
Metabolism
Etoricoxib is metabolized involving oxidation of its 6′-methyl group primarily by CYP3A4 but is not an inhibitor of CYP3A4. Other metabolites include 1′-N-oxide and glucuronides. Etoricoxib is primarily excreted as metabolites into the urine.
Properties of Etoricoxib
| Melting point: | 134-135°C |
| Boiling point: | 510.0±50.0 °C(Predicted) |
| Density | 1.298±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO : 100 mg/mL (278.68 mM; Need ultrasonic) |
| pka | 4.5(at 25℃) |
| form | neat |
| form | Solid |
| color | Light yellow to yellow |
| BRN | 8073797 |
| CAS DataBase Reference | 202409-33-4(CAS DataBase Reference) |
Safety information for Etoricoxib
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H310:Acute toxicity,dermal |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Etoricoxib
| InChIKey | MNJVRJDLRVPLFE-UHFFFAOYSA-N |
Etoricoxib manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Etoricoxib 99%View Details
Etoricoxib 99%View Details -
 ETORICOXIB 99%View Details
ETORICOXIB 99%View Details -
 Etoricoxib 98%View Details
Etoricoxib 98%View Details -
 Etoricoxib 98%View Details
Etoricoxib 98%View Details -
 Etoricoxib 98%View Details
Etoricoxib 98%View Details -
 Etoricoxib 98%View Details
Etoricoxib 98%View Details -
 Etoricoxib 99%View Details
Etoricoxib 99%View Details -
 ETORICOXIB 99%View Details
ETORICOXIB 99%View Details
