Eugenol
Synonym(s):2-Methoxy-4-(2-propenyl)phenol;4-Allyl-2-methoxyphenol;4-Allylguaiacol;Eugenol;Eugenolum
- CAS NO.:97-53-0
- Empirical Formula: C10H12O2
- Molecular Weight: 164.2
- MDL number: MFCD00008654
- EINECS: 202-589-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
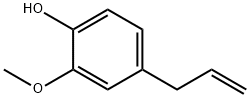
What is Eugenol?
Description
Sensitization to eugenol mainly occurs in those in dental professions. Eugenol is contained in the "fragrance mix".
Description
The spicy fragrance of cloves comes largely from eugenol, the principal ingredient in oil of cloves. Eugenol is used not only in perfumes and mouthwashes, but also as an insect attractant and a dental analgesic. It can be oxidized to form vanillin, which has a vanilla scent.
Chemical properties
colourless to faintly yellow liquid with a strong odour of cloves
Chemical properties
Eugenol is the main component
of several essential oils; clove leaf oil and cinnamon leaf oilmay contain>90%.Eugenol occurs in small amounts in many other essential oils. It is a colorless to
slightly yellow liquid with a spicy, clove odor.
Catalytic hydrogenation (e.g., in the presence of noble metal catalysts) yields
dihydroeugenol. Isoeugenol is obtained fromeugenol by shifting the double bond.
Esterification and etherification of the hydroxy group of eugenol yield valuable
fragrance and flavor materials (e.g., eugenol acetate and eugenol methyl ether).
Chemical properties
Eugenol has a strong aromatic odor of clove and a spicy, pungent taste. It darkens and thickens on exposure to air.
Occurrence
Reported found as a constituent in several volatile oils: clove oil, laurel and cinnamon leaf oil. Smaller amounts of eugenol are also present in the oil of camphor, Java citronella, California laurel and acacia flowers; remarkable amounts of eugenol are found in Ocimum sanctum (70%) and Ocimum gratissimum (60%). Eugenol is also found in the oil from violet flowers (21%); in some plants, eugenol probably occurs as glucoside. Reported found in apricot, citrus oils, raspberry, strawberry, tomato, anise, cinnamon (leaf, bark and roots), clove bud and stem, nutmeg, mace, pepper, smoked fish, beer, whiskey, grape wines, cocoa, mango, tarragon, laurel, myrtle leaf, and pimento berry and leaf.
The Uses of Eugenol
Eugenol is a dental compound which shows cytotoxicity to human oral squamous cell carcinoma and oral cells. When glucosylated, this compound exhibits anti-inflammatory activity.
The Uses of Eugenol
analgesic (topical), antiseptic, antifungal
The Uses of Eugenol
blood volume expander
The Uses of Eugenol
eugenol is a botanical fraction. It is anti-bacterial, anti-inflammatory, and pain relieving. It can also be used as a local, topical anesthetic and antiseptic. In a cosmetic formulation, it can mask odor or provide fragrance. eugenol is a yellow, oily liquid and is generally associated with clove oil. However, it is also found in nutmeg, cinnamon, and bay leaf.
The Uses of Eugenol
Eugenol is a flavoring obtained from clove oil and also found in car- nation and cinnamon leaves. it is a stable, light yellow-green liquid of clove odor. it is slightly soluble in water and miscible in alcohol. it should be stored in glass or tin, avoiding iron containers. it is used in spice oils for application in condiments and meats at 100–200 ppm and in baked goods and candy at approximately 30 ppm.
Background
Eugenol is a naturally occurring phenolic molecule found in several plants such as cinnamon, clove, and bay leaves. It has been used as a topical antiseptic as a counter-irritant and in dental preparations with zinc oxide for root canal sealing and pain control. Although not currently available in any FDA-approved products (including OTC), eugenol has been found to have anti-inflammatory, neuroprotective, antipyretic, antioxidant, antifungal and analgesic properties. Its exact mechanism of action is unknown, however, it has been shown to interfere with action potential conduction. There are a number of unapproved OTC products available containing eugenol that advertise its use for the treatment of toothache.
Indications
Eugenol is not currently available in any FDA-approved drug products. There are a number of unapproved OTC products that advertise it for the use of toothache. Eugenol is is also commonly used in combination with zinc oxide in dental procedures for the cementation of temporary prostheses and the temporary restoration of teeth and cavities.
What are the applications of Application
Eugenol is an antifungal antimicrobial agonist of the TRPV1 receptor
Definition
ChEBI: A phenylpropanoid formally derived from guaiacol with an allyl chain substituted para to the hydroxy group.
Preparation
Since sufficient eugenol can be isolated from cheap essential oils, synthesis is not industrially important. Eugenol is still preferentially isolated from clove leaf and cinnamon leaf oil (e.g., by extraction with sodium hydroxide solution). Nonphenolic materials are then removed by steam distillation. After the alkaline solution is acidified at low temperature, pure eugenol is obtained by distillation.
Aroma threshold values
Detection: 6 to 100 ppb
General Description
Clear colorless pale yellow or amber-colored liquid. Odor of cloves. Spicy pungent taste.
Air & Water Reactions
Darkens and thickens on exposure to air. Also darkens with age. Eugenol may decompose on exposure to light. Insoluble in water.
Reactivity Profile
Eugenol is incompatible with strong oxidizers. This includes ferric chloride and potassium permanganate. Eugenol reacts with strong alkalis. Eugenol is incompatible with iron and zinc.
Hazard
Questionable carcinogen.
Fire Hazard
Eugenol is combustible.
Contact allergens
Eugenol is a fragrance allergen obtained from many natural sources. Occupational sensitization to eugenol may occur in dental profession workers. Eugenol is contained in “fragrance mix” and has to be listed by name in cosmetics within the EU.
Anticancer Research
This compound was tested on a model of skin tumor induced by DMBA croton oilin Swiss mice. The eugenol affects the cellular proliferation by increasing apoptosiscellular death. There is evidence for a downregulation of c-myc, H-ras, and Bcl-2expression and an upregulation of p53, Bax, and active caspase-3 (Grondona et al.2014).
Clinical Use
4-Allyl-2-methoxyphenol is obtained primarily from cloveoil. It is a pale-yellow liquid with a strong aroma of clovesand a pungent taste. Eugenol is only slightly soluble in waterbut is miscible with alcohol and other organic solvents.Eugenol possesses both local anesthetic and antiseptic activityand can be directly applied on a piece of cotton to relievetoothaches. Eugenol is also used in mouthwashes because ofits antiseptic property and pleasant taste. The phenol coefficientof eugenol is 14.4.
Safety Profile
Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. Human mutation data reported. A human skin irritant. Questionable carcinogen with experimental carcinogenic and tumorigenic data. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALLYL COMPOUNDS.
Synthesis
The oil containing eugenol is treated with a 3% aqueous solution of NaOH; the nonacid components are extracted with ether; the alkaline solution is acidified to isolate the phenols and subsequently is fractionally distilled under reduced pressure; to avoid the formation of emulsions, a pretreatment of the oil with tartaric acid is preferred; eugenol is the starting material in one of the syntheses for the preparation of vanillin.
Metabolism
Not Available
Metabolism
No absorption of eugenol occurred within 2hr of application to the intact shaved skin of mice (Meyer & Meyer, 1959). Following ip injec tion of [14C]eugenol into rats, radioactivity was dis tributed in various organs and the presence of 14CO2 in the expired air indicated the demethylation of eugenol (Weinberg, Rabinowitz, Zanger & Gennaro, 1972). Over 70% of an oral dose of eugenol was excreted in the urine of rabbits (Schr?der & Vollmer, 1932).
Purification Methods
Fractional distillation of eugenol gives a pale yellow liquid which darkens and thickens on exposure to air. It should be stored under N2 at -20o. [Waterman & Priedster Recl Trav Chim Pays-Bas 48 1272 1929, Beilstein 6 H 961, 6 IV 6337.]
Properties of Eugenol
| Melting point: | -12--10 °C (lit.) |
| Boiling point: | 254 °C (lit.) |
| Density | 1.067 g/mL at 25 °C (lit.) |
| vapor pressure | <0.1 hPa (25 °C) |
| FEMA | 2467 | EUGENOL |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | 2.46g/l |
| form | Liquid |
| pka | pKa 9.8 (Uncertain) |
| color | Clear pale yellow to yellow |
| Odor | at 10.00 % in dipropylene glycol. sweet spicy clove woody |
| Water Solubility | slightly soluble |
| Sensitive | Air Sensitive |
| Merck | 14,3898 |
| JECFA Number | 1529 |
| BRN | 1366759 |
| Dielectric constant | 6.1(18℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 97-53-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Eugenol(97-53-0) |
| IARC | 3 (Vol. 36, Sup 7) 1987 |
| EPA Substance Registry System | Eugenol (97-53-0) |
Safety information for Eugenol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Eugenol
| InChIKey | RRAFCDWBNXTKKO-UHFFFAOYSA-N |
Eugenol manufacturer
New Products
Tetrabutylammonium iodide (3,3-DIFLUOROCYCLOBUTYL)METHANOL 4,4-DIFLUOROCYCLOHEXANAMINE Cyclobutylamine (S)-3-Fluoro-pyrrolidine hydrochloride 3-Oxocyclobutanecarboxylic acid N-Hydroxy-2-methylpropanimidamide L-tert-Leucine,97% 2-Bromophenylacetonitrile, 97% Aluminum oxide, basic Calcium hydroxide, 95% Diallylamine, 99% 2-Iodobenzoic Acid 3-Methoxybenzonitrile Pentachlorobenzonitrile Chloral Dibenzoyl Peroxide Titanium Dioxide O-Benzylhydroxylamine Hydrochloride 2-Nitrobenzaldehyde 2-Picolylamine (2-Aminomethylpyridine) 2-Venylpyridine Ethyl-2-Chloroacetoacetate 4-Dimethylamine PyridineRelated products of tetrahydrofuran



You may like
-
 Eugenol 99%View Details
Eugenol 99%View Details
97-53-0 -
 Eugenol extrapure CAS 97-53-0View Details
Eugenol extrapure CAS 97-53-0View Details
97-53-0 -
 Eugenol 98.00% CAS 97-53-0View Details
Eugenol 98.00% CAS 97-53-0View Details
97-53-0 -
 Eugenol CAS 97-53-0View Details
Eugenol CAS 97-53-0View Details
97-53-0 -
 Eugenol CAS 97-53-0View Details
Eugenol CAS 97-53-0View Details
97-53-0 -
 Eugenol CAS 97-53-0View Details
Eugenol CAS 97-53-0View Details
97-53-0 -
 Eugenol CAS 97-53-0View Details
Eugenol CAS 97-53-0View Details
97-53-0 -
 EUGENOL Extra Pure CAS 97-53-0View Details
EUGENOL Extra Pure CAS 97-53-0View Details
97-53-0