Probenecid
Synonym(s):p-(Dipropylsulfamoyl)benzoic acid;Probenecid
- CAS NO.:57-66-9
- Empirical Formula: C13H19NO4S
- Molecular Weight: 285.36
- MDL number: MFCD00038402
- EINECS: 200-344-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
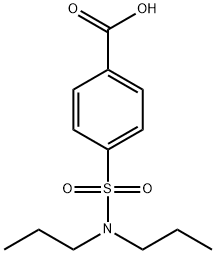
What is Probenecid?
Description
Probenecid is insoluble in water and acidic solutions but is soluble in alkaline solutions buffered to pH 7.4. Probenecid initially was synthesized as a result of studies in the 1940s on sulfonamides that indicated the sulfonamides decreased the renal clearance of penicillin, extending the half-life of penicillin as supplies diminished. Probenecid thus was initially used—and is still indicated—for that purpose. Probenecid promotes the excretion of uric acid by inhibiting the urate anion exchange transporter (URAT 1), decreasing the reabsorption of uric acid in the proximal tubules. The overall effect is to decrease plasma uric acid concentrations, thereby decreasing the rate and extent of urate crystal deposition in joints and synovial fluids. Within the series of N-dialkylsulfamyl benzoates from which probenecid is derived, renal clearance of these compounds is decreased as the length of the N-alkyl substituents is increased. Uricosuric activity increases with increasing size of the alkyl group in the series methyl, ethyl, and propyl.
Chemical properties
White to Off-White Solid
The Uses of Probenecid
It is a uricosuric drug, that is, it increases uric acid excretion in the urine. It is primarily used in treating gout and hyperuricemia. Probenecid was developed as an alternative to caronamide to competitively inhibit renal excretion of some drugs, thereby increasing their plasma concentration and prolonging their effects.
The Uses of Probenecid
Uricosuric drug.
The Uses of Probenecid
pharmaceutical intermediate
The Uses of Probenecid
For the reduction of serum uric acid concentrations in chronic gouty arthritis and tophaceous gout in patients with frequent disabling gout attacks. Has also been effectively used to promote uric acid excretion in hyperuricemia secondary to the administra
The Uses of Probenecid
An inhibitor of several ABC-transporters of the subfamily ABCC or MRP.
Background
The prototypical uricosuric agent. It inhibits the renal excretion of organic anions and reduces tubular reabsorption of urate. Probenecid has also been used to treat patients with renal impairment, and, because it reduces the renal tubular excretion of other drugs, has been used as an adjunct to antibacterial therapy.
Indications
For the reduction of serum uric acid concentrations in chronic gouty arthritis and tophaceous gout in patients with frequent disabling gout attacks. Has also been effectively used to promote uric acid excretion in hyperuricemia secondary to the administration of thiazide and related diuretics.
What are the applications of Application
Probenecid is an inhibitor of several ABC-transporters of the subfamily ABCC or MRP
Definition
ChEBI: A sulfonamide in which the nitrogen of 4-sulfamoylbenzoic acid is substituted with two propyl groups.
Indications
When probenecid (ColBENEMID) is given in sufficient amounts, it will block the active reabsorption of uric acid in the proximal tubules following its glomerular filtration, thereby increasing the amount of urate eliminated. In contrast, low dosages of probenecid appear to compete preferentially with plasma uric acid for the proximal tubule anionic transport system and thereby block its access to this active secretory system. The uricosuric action of probenecid, however, is accounted for by the drug’s ability to inhibit the active reabsorption of filtered urate.
brand name
Benemid (Merck); Probalan (Lannett).
General Description
Odorless white or almost white crystalline powder. Slightly bitter taste; pleasant aftertaste.
General Description
Probenecid (Benemid) is the most widely used uricosuricagent in the United States. It is selectively excreted into therenal tubules by OATS. It is extensively metabolized via Ndealkylationor ω-oxidation, followed by phase II conjugationinto the active metabolite, p-sulfamyl hippurate, whichexhibits a high affinity, similar to p-aminohippurate, forbinding to OATS, thereby preventing uric acid reabsorptionfrom the renal proximal tubules.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Probenecid may be light sensitive .
Fire Hazard
Flash point data for Probenecid are not available. Probenecid is probably combustible.
Mechanism of action
Probenecid is rapidly absorbed after oral administration, with peak plasma levels usually reached in 2 to 4 hours. Its half-life is somewhat variable (6–12 hours) because of both its extensive plasma protein binding and its active proximal tubular secretion. Since tubular backdiffusion is decreased at alkaline urinary pH ranges, probenecid excretion increases with increasing urinary pH. Probenecid is rapidly metabolized, with less than 5% of an administered dose being eliminated in 24 hours.The major metabolite is an acyl monoglucuronide.
Pharmacokinetics
Probenecid is a uricosuric and renal tubular blocking agent and is used in combination with colchicine to treat chronic gouty arthritis when complicated by frequent, recurrent acute attacks of gout. It inhibits the reabsorption of urate at the proximal convoluted tubule, thus increasing the urinary excretion of uric acid and decreasing serum urate levels. Effective uricosuria reduces the miscible urate pool, retards urate deposition, and promotes resorption of urate deposits. At the proximal and distal tubles, probenecid competitively inhibits the secretion of many weak organic acids including penicillins, most cephalosporins, and some other β-lactam antibiotics. This results in an increase in the plasma concentrations of acidic drugs eliminated principally by renal secretion, but only a slight increase if the drug is eliminated mainly by filtration. Thus, the drug can be used for therapeutic advantages to increase concentrations of certain β-lactam antibiotics in the treatment of gonorrhea, neurosyphilis, or pelvic inflammatory disease (PID).
Pharmacokinetics
Probenecid is essentially completely absorbed from the GI tract on oral administration, with peak plasma levels observed within 2 to 4 hours. Like most acidic compounds, probenecid (pKa = 3.4) is extensively plasma protein bound (93–99%). The primary route of elimination of probenecid and its metabolites is the urine. It is extensively metabolized in humans, with only 5 to 10% being excreted as unchanged drug. The major metabolites detected result from glucuronide conjugation of the carboxylic acid, ω-oxidation of the n-propyl side chain and subsequent oxidation of the resulting alcohol to the carboxylic acid derivative, ω1-oxidation of the n-propyl group, and N-dealkylation.
Clinical Use
Probenecid is an effective and relatively safe agent
for controlling hyperuricemia and preventing tophi
deposition in tissues. Chronic administration will decrease
the incidence of acute gouty attacks as well as diminish
the complications usually associated with hyperuricemia,
such as renal damage and tophi deposition.
Probenecid is still used by some physicians to maintain
high blood levels of penicillin, cephalosporin, acyclovir,
and cyclosporine. It is not useful in treating acute attacks
of gouty arthritis. If the total amount of uric acid
excreted is greater than 800 mg/day, the urine should be
alkalinized to prevent kidney stone formation and promote
uric acid.
Side Effects
The major side effect is GI distress (e.g., nausea, vomiting, and anorexia), but these occur in only 2% of patients at low doses. Other effects include headache, dizziness, urinary frequency, hypersensitivity reactions, sore gums, and anemia.
Veterinary Drugs and Treatments
Although there has been very limited clinical use or research on
probenecid in veterinary medicine, it can be useful in treating gout
(hyperuricemia), particularly in reptiles.
Probenecid’s effect in inhibiting renal tubular secretion of certain
beta-lactam antibiotics and other weak organic acids is of interest
for increasing serum concentrations, or reducing doses and dosing
frequency of these drugs. This may allow greater efficacy (but also
toxic effects) and reduce the cost or dosing frequency of expensive
human drugs. Probenecid has a significantly long elimination halflife
in dogs (about 18 hours), which may make it particularly useful
in this species; however, at present there is little research supporting
this use of probenecid in veterinary patients.
Metabolism
Not Available
storage
Store at RT
Properties of Probenecid
| Melting point: | 194-196°C |
| Boiling point: | 438.0±47.0 °C(Predicted) |
| Density | 1.2483 (rough estimate) |
| refractive index | 1.6800 (estimate) |
| storage temp. | Store at RT |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | neat |
| pka | 5.8(at 25℃) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | <0.1 g/100 mL at 20 ºC |
| Merck | 14,7754 |
| Stability: | Stable, but may be light sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 57-66-9(CAS DataBase Reference) |
| NIST Chemistry Reference | P-(dipropylsulfamoyl) benzoic acid(57-66-9) |
| EPA Substance Registry System | Probenecid (57-66-9) |
Safety information for Probenecid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Probenecid
Probenecid manufacturer
DK Pharmachem Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 3-Nitrobenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 57-66-9 Probenecid 99%View Details
57-66-9 Probenecid 99%View Details
57-66-9 -
 57-66-9 98%View Details
57-66-9 98%View Details
57-66-9 -
 Probenecid 98% CAS 57-66-9View Details
Probenecid 98% CAS 57-66-9View Details
57-66-9 -
 Probenecid CAS 57-66-9View Details
Probenecid CAS 57-66-9View Details
57-66-9 -
 Probenecid CAS 57-66-9View Details
Probenecid CAS 57-66-9View Details
57-66-9 -
 Probenecid CAS 57-66-9View Details
Probenecid CAS 57-66-9View Details
57-66-9 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0