1,2-Dimethoxyethane
Synonym(s):DME;1,2-Dimethoxyethane;Monoglyme;Ethylene glycol dimethyl ether;Ethylene glycol dimethyl ether solution
- CAS NO.:110-71-4
- Empirical Formula: C4H10O2
- Molecular Weight: 90.12
- MDL number: MFCD00008502
- EINECS: 203-794-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
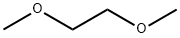
What is 1,2-Dimethoxyethane?
Description
1,2-Dimethoxyethane (DME) is a liquid ether used as an aprotic solvent. It is also known as glyme, monoglyme, dimethyl glycol, ethylene glycol, dimethyl ether, dimethyl cellosolve, and DME. It has the special ability to form chelates thanks to the high flexibility of its chains. Moreover, DME is the molecular model system for the poly(oxyethylene) chain. It is commonly used as a solvent in batteries and in organometallic reaction chemistry since it has higher boiling than diethyl ether and THF[1].
Chemical properties
Ethylene glycol dimethyl ether, also called 1,2-Dimethoxyethane, is a colorless liquid with a strong smell similar to ether. It is stable and does not easily react. It can dissolve different resins and cellulose, and can be mixed with water and various organic solvents like alcohols, ketones, and esters in any amount. Its solubility can be changed by diluting it with water or specific solvents.
The Uses of 1,2-Dimethoxyethane
Solvent commonly employed in organometallic reactions,1 particularly organolithium reactions.2 May also function as a ligand.3
The Uses of 1,2-Dimethoxyethane

The SM (39.4 g, 149.98 mmol) and DIEA (51.69 mL, 300 mmol) were dissolved in THF (550 mL) and stirred at -10 C under N2. Then a solution of isobutylchloroformate in THF (50 mL) was added dropwise and stirring was continued for 1 h at -10 C, then 1 h at RT. NaBH4 (17.02 g, 450 mmol) was added portionwise at -10 C and stirred for 1 h. H2O (200 mL) was added cautiously to the reaction mixture and stirring was continued for another hour at RT under N2. The mixture was neutralized with 10% citric acid (aq) and then was extracted with EtOAc. The org layer was dried (MgSO4) and concentrated. The residue was purified by silica gel chromatography (50:50:0 to 0:100:0 to 0:99:1 heptane/DCM/MeOH) to provide the product as a white powder. [23.9 g, 64%]
The Uses of 1,2-Dimethoxyethane
1,2-Dimethoxyethane is widely used as a solvent for electrolyte of lithium batteries, polysilicones, oligo- and polysaccharides. It plays an important role in Grignard reactions, Suzuki reactions and Stille couplings in organometallic chemistry and in pharmaceutical synthesis. It is a higher boiling point solvent and is used as an alternative to diethyl ether and tetrahydrofuran. It is used for the etching of synthetic polymers like polytetrafluoroethylene and other fluoropolymers with alkali metal dispersions.
What are the applications of Application
1,2-Dimethoxyethane is a solvent used in the formation of alikalai metal-hydro-carbon adducts
Definition
ChEBI: 1,2-dimethoxyethane is a diether that is the 1,2-dimethyl ether of ethane-1,2-diol. It has a role as a non-polar solvent. It is functionally related to an ethylene glycol.
What are the applications of Application
Monoglyme serves as an electrolyte solution component for lithium batteries. It is also frequently utilized as a solvent in organometallic reactions, particularly those involving organolithium compounds. Additionally, it can act as a ligand in certain chemical reactions.
General Description
A liquid with a sharp odor. Less dense than water. Flash point 34°F. Mildly toxic by ingestion and inhalation. Severely irritates skin and eyes. Vapors heavier than air. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
When the solvent, 1,2-Dimethoxyethane, was poured into a funnel previously used to introduce the lithium aluminum hydride, a fire ignited the funnel, [MCA Case History 1182(1966)].
Hazard
Moderate fire risk.
Health Hazard
If ingested causes nausea, vomiting, cramps, weakness, coma.
Fire Hazard
Behavior in Fire: Containers may explode in fires.
Safety Profile
An experimental teratogen. Other experimental reproductive effects. Readdy forms an explosive peroxide. A very dangerous fire hazard when exposed to heat, flame, or oxidzers. Mixture with lithium tetrahydroaluminate may ignite orexplode if heated. When heated to decomposition it emits acrid smoke and fumes. See also GLYCOL ETHERS.
Synthesis
1,2-Dimethoxyethane is derived from the reaction of ethylene glycol monomethyl ether with sodium metal and methyl chloride. The ethylene glycol monomethyl ether and the metal sodium were refluxed together until the metal sodium was completely reacted, the temperature was lowered to 45° C., and methyl chloride was introduced. After the reaction is completed, fractional distillation is performed to collect fractions at 84-85.5°C to obtain 1,2-Dimethoxyethane.
Purification Methods
Traces of water and acidic materials have been removed from it by refluxing with Na, K or CaH2, decanting and distilling from Na, K, CaH2 or LiAlH4. The reaction has been speeded up by using vigorous high-speed stirring with molten potassium. For virtually complete elimination of water, 1,2-dimethoxyethane has been dried with Na-K alloy until a characteristic blue colour is formed in the solvent at Dry-ice/cellosolve temperatures: the solvent is kept with the alloy until distilled for use [Ward J Am Chem Soc 83 1296 1961]. Alternatively, glyme, refluxed with benzophenone and Na-K, is dry enough if, on distillation, it gives a blue colour of the ketyl immediately on addition to benzophenone and sodium [Ayscough & Wilson J Chem Soc 5412 1963]. It has also been purified by distillation under N2 from sodium benzophenone ketyl (see above). [Beilstein 1 IV 2376.]
References
[1] Weixing Li . “The microwave spectroscopy study of 1,2-dimethoxyethane.” Journal of Molecular Spectroscopy 337 (2017): Pages 3-8.
Properties of 1,2-Dimethoxyethane
| Melting point: | -69 °C |
| Boiling point: | 85 °C(lit.) |
| Density | 0.867 g/mL at 25 °C(lit.) |
| vapor density | 3.1 (20 °C, vs air) |
| vapor pressure | 48 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 32 °F |
| storage temp. | Store below +30°C. |
| solubility | 1000g/l soluble |
| appearance | Colorless liquid |
| form | Liquid |
| color | Clear |
| Odor | water-wh. liq., sharp ethereal odor |
| explosive limit | 10.4% |
| Water Solubility | Miscible |
| Sensitive | Air Sensitive |
| λmax | λ: 220 nm Amax: 1.00 λ: 250 nm Amax: 0.20 λ: 300 nm Amax: 0.03 λ: 350-400 nm Amax: 0.01 |
| Merck | 14,3224 |
| BRN | 1209237 |
| Dielectric constant | 7.2(25℃) |
| Stability: | Stable, but explosive peroxides may form on exposure to air. Test for the presence of peroxides before heating. Avoid heat, light, air. Highly flammable. Store under inert gas. |
| CAS DataBase Reference | 110-71-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethane, 1,2-dimethoxy-(110-71-4) |
| EPA Substance Registry System | Ethylene glycol dimethyl ether (110-71-4) |
Safety information for 1,2-Dimethoxyethane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 1,2-Dimethoxyethane
| InChIKey | XTHFKEDIFFGKHM-UHFFFAOYSA-N |
1,2-Dimethoxyethane manufacturer
JSK Chemicals
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 MONOGLYME 99%View Details
MONOGLYME 99%View Details -
 1,2-Dimethoxyethane (Monoglyme CAS 110-71-4View Details
1,2-Dimethoxyethane (Monoglyme CAS 110-71-4View Details
110-71-4 -
 1,2-Dimethoxyethane (Monoglyme CAS 110-71-4View Details
1,2-Dimethoxyethane (Monoglyme CAS 110-71-4View Details
110-71-4 -
 1,2-Dimethoxyethane CAS 110-71-4View Details
1,2-Dimethoxyethane CAS 110-71-4View Details
110-71-4 -
 Monoglyme CASView Details
Monoglyme CASView Details -
 Monoglyme CASView Details
Monoglyme CASView Details -
 Monoglyme Liquid Chemical CAS 110-71-4View Details
Monoglyme Liquid Chemical CAS 110-71-4View Details
110-71-4 -
 Liquid 1,2-Dimethoxyethane, Packaging Size: Fiber DrumView Details
Liquid 1,2-Dimethoxyethane, Packaging Size: Fiber DrumView Details
110-71-4
