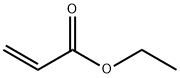
Ethyl pyruvate
Synonym(s):Ethyl pyruvate;Pyruvic acid ethyl ester, Ethyl 2-oxopropanoate, Ethyl 2-oxopropionate
- CAS NO.:617-35-6
- Empirical Formula: C5H8O3
- Molecular Weight: 116.12
- MDL number: MFCD00009123
- EINECS: 210-511-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
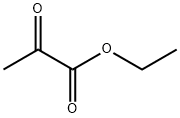
What is Ethyl pyruvate?
Chemical properties
clear pale yellow liquid
Chemical properties
Ethyl pyruvate has a vegetable, caramel odor.
Occurrence
Reported found in Parmesan cheese, cognac, grape wines, cocoa and mushrooms.
The Uses of Ethyl pyruvate
Ethyl pyruvate (EP) has demonstrated neuroprotective effects against acute brain injury through its anti-inflammatory action.
The Uses of Ethyl pyruvate
Ethyl pyruvate is used as a flavoring agent in food and anti-inflammatory agent for the treatment of critical inflammatory conditions. It is used in the treatment of critical illnesses such as severe sepsis, acute respiratory distress syndrome, burn injury, acute pancreatitis and stroke. It plays an important role in cardiac function after coronary ischemia and reperfusion.
The Uses of Ethyl pyruvate
Ethyl pyruvate can undergo asymmetric Henry reaction with nitromethane to form α-hydroxy β-nitro esters. It can be used as the model compound for α-ketoesters to study the mechanism of enantioselective hydrogenation reactions.
What are the applications of Application
Ethyl pyruvate is an organic building block that is a novel anti inflammatory in vitro
Preparation
By direct esterification of pyruvic acid with absolute ethyl alcohol at the boil and subsequent vacuum distillation; by esterification via oxidation of vapors of ethyl lactate in the presence of V2O5 at 155°C
Definition
ChEBI: Ethyl pyruvate is an oxo carboxylic acid.
Taste threshold values
Taste characteristics at 60 ppm: sweet, rum-like with a fruity ethereal nuance.
Synthesis Reference(s)
Tetrahedron Letters, 34, p. 7191, 1993 DOI: 10.1016/S0040-4039(00)79284-7
General Description
Ethyl pyruvate is a volatile aliphatic ester of the pyruvate metabolite. It is present as a food additive/odor-active component in various food and beverage products. It is also used as an inhibitory odorant to repel the insects from human proximity.
Purification Methods
Shake the ester with 10mL portions of saturated aqueous CaCl2 solution (removes ethyl acetate) and the organic layer is removed by centrifugation, decantation and filtration, and is distilled under reduced pressure. Purification of small quantities is carried out via the bisulfite adduct: the ester (2.2mL) is shaken with saturated NaHSO3 (3.6mL), chilled in a freezing mixture when crystals separate rapidly (particularly if seeded). After 5minutes EtOH (10mL) is added and the crystals are filtered off, washed with EtOH and Et2O and dried. Yield ca 3g of bisulfite adduct. Then treat the adduct (16g) with saturated aqueous MgSO4 (32mL) and 40% formaldehyde (5mL) and shake, whereby the ester separates as an oil which is extracted with Et2O. The extract is dried (MgSO4), filtered, evaporated and the residue is distilled (b 56o/20mm), and then redistilled (b 147.5o/750mm) to give 5.5g of pure ester. [Cornforth Org Synth Coll Vol IV 467 1963, Beilstein 3 IV 1513.]
Properties of Ethyl pyruvate
| Melting point: | -58 °C |
| Boiling point: | 144 °C (lit.) |
| Density | 1.045 g/mL at 25 °C (lit.) |
| vapor pressure | 2.36hPa at 25℃ |
| FEMA | 2457 | ETHYL PYRUVATE |
| refractive index | n |
| Flash point: | 114 °F |
| storage temp. | Store at +2°C to +8°C. |
| solubility | 10g/l |
| form | Liquid |
| color | Clear pale yellow |
| Odor | at 10.00 % in dipropylene glycol. ether fruity sweet sharp rum vegetable caramel |
| Water Solubility | Miscible with water, ethanol and ether. |
| JECFA Number | 938 |
| Merck | 14,8021 |
| BRN | 1071466 |
| Stability: | Volatile |
| CAS DataBase Reference | 617-35-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Propanoic acid, 2-oxo-, ethyl ester(617-35-6) |
| EPA Substance Registry System | Propanoic acid, 2-oxo-, ethyl ester (617-35-6) |
Safety information for Ethyl pyruvate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H226:Flammable liquids H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ethyl pyruvate
| InChIKey | XXRCUYVCPSWGCC-UHFFFAOYSA-N |
Ethyl pyruvate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Ethyl pyruvate 99%View Details
Ethyl pyruvate 99%View Details -
 Ethyl Pyruvate CASView Details
Ethyl Pyruvate CASView Details -
 Ethyl pyruvate CAS 617-35-6View Details
Ethyl pyruvate CAS 617-35-6View Details
617-35-6 -
 Ethyl Pyruvate CAS 617-35-6View Details
Ethyl Pyruvate CAS 617-35-6View Details
617-35-6 -
 Ethyl pyruvate 98% (GC) CAS 617-35-6View Details
Ethyl pyruvate 98% (GC) CAS 617-35-6View Details
617-35-6 -
 Ethyl pyruvate 97% CAS 617-35-6View Details
Ethyl pyruvate 97% CAS 617-35-6View Details
617-35-6 -
 Ethyl Pyruvate (617-35-6 ), Pack Type: DrumView Details
Ethyl Pyruvate (617-35-6 ), Pack Type: DrumView Details
617-35-6 -
 Ethyl Pyruvate 617-35-6View Details
Ethyl Pyruvate 617-35-6View Details
617-35-6
