Ethyl bromodifluoroacetate
- CAS NO.:667-27-6
- Empirical Formula: C4H5BrF2O2
- Molecular Weight: 202.98
- MDL number: MFCD00042069
- EINECS: 211-567-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
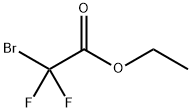
What is Ethyl bromodifluoroacetate?
Description
Ethyl bromodifluoroacetate, a clear to yellow liquid, is an ester of bromodifluoroacetic acid and ethyl alcohol. It is an ester that can be used to introduce the CF2 group when synthesizing chemical compounds. Ethyl bromdifluoroacetate is considered to be a good compound in the generation of compounds and for testing with other organic compounds like lactones, imines, and other amino acids.
Chemical properties
clear colorless to slightly yellow liquid
The Uses of Ethyl bromodifluoroacetate
Ethyl bromodifluoroacetate is usually used in the preparation of substituted ethyl 2′-pyridyldifluoroacetates,4-alkoxymethyl- and 4-aryloxymethyl-3,3-difluoropiperidines,α,α-difluoro-β-lactams,mono- and difluoromethylated phenanthridine derivatives.
The Uses of Ethyl bromodifluoroacetate
Efficient difluoroalkylation of aryl boronic acids via palladium catalysis. Novel, moisture-stable bromodifluoromethylated phosphonate, ester, and amide. Efficient coupling with aryl boronic acids via Pd catalysis. A Cu-catalyzed regioselective bromodifluoroacetylation of alkenes, using ethyl bromodifluoroacetate (BrCF2CO2Et) as a difluoroacetylating reagent.
The Uses of Ethyl bromodifluoroacetate
Ethyl bromodifluoroacetate, is used for the treatment of the Reformat sky reagent with aldehydes and ketones affords 2,2-difluoro-3-hydroxy esters. It is also used in the analysis of reagent purity through IR, NMR.
Preparation
Difluorobromoacetonitrile is taken as a raw material, and prepared through a one-pot method and subjected to alcoholysis and esterification in ethanol aqueous solution to obtain ethyl bromodifluoroacetate.Reaction equation is:
BrF2CCN→BrF2CCOOH→BrF2CCOOC2H5
Properties of Ethyl bromodifluoroacetate
| Boiling point: | 112 °C/700 mmHg (lit.) |
| Density | 1.583 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 70 °F |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Soluble in most organic solvents. |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| Specific Gravity | 1.583 |
| BRN | 1906095 |
| InChI | InChI=1S/C4H5BrF2O2/c1-2-9-3(8)4(5,6)7/h2H2,1H3 |
| CAS DataBase Reference | 667-27-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethyl bromodifluoroacetate(667-27-6) |
Safety information for Ethyl bromodifluoroacetate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H225:Flammable liquids H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ethyl bromodifluoroacetate
| InChIKey | IRSJDVYTJUCXRV-UHFFFAOYSA-N |
| SMILES | C(OCC)(=O)C(Br)(F)F |
Ethyl bromodifluoroacetate manufacturer
JSK Chemicals
Gujarat Fluorochemicals Limited (GFL)
ASM Organics
Related products of tetrahydrofuran

You may like
-
 Ethyl bromodifluoroacetate 98%View Details
Ethyl bromodifluoroacetate 98%View Details -
 Ethyl bromodifluoroacetate 667-27-6 98%View Details
Ethyl bromodifluoroacetate 667-27-6 98%View Details
667-27-6 -
 Ethyl bromodifluoroacetate CAS 667-27-6View Details
Ethyl bromodifluoroacetate CAS 667-27-6View Details
667-27-6 -
 Ethyl bromodifluoroacetate, 98% CAS 667-27-6View Details
Ethyl bromodifluoroacetate, 98% CAS 667-27-6View Details
667-27-6 -
 Ethyl Bromodifluoroacetate CAS 667-27-6View Details
Ethyl Bromodifluoroacetate CAS 667-27-6View Details
667-27-6 -
 Ethyl bromodifluoroacetate, 98% 667-27-6 99%View Details
Ethyl bromodifluoroacetate, 98% 667-27-6 99%View Details
667-27-6 -
 Ethyl bromodifluoroacetate 98% CASView Details
Ethyl bromodifluoroacetate 98% CASView Details -
 Ethyl bromodifluoroacetate CAS 667-27-6View Details
Ethyl bromodifluoroacetate CAS 667-27-6View Details
667-27-6