Estriol
Synonym(s):1,3,5(10)-Estratriene-3,16α,17β-triol;16α-Hydroxyestradiol;3,16α,17β-Trihydroxy-1,3,5(10)-estratriene;Estriol
- CAS NO.:50-27-1
- Empirical Formula: C18H24O3
- Molecular Weight: 288.38
- MDL number: MFCD00003691
- EINECS: 200-022-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
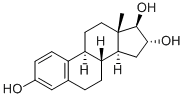
What is Estriol?
Toxicity
ORAL (LD50): Acute: >2000 mg/kg [Rat].
Description
Estriol is significantly less active than estradiol; however, it has a selective ability to stimulate blood flow and restoration of genital epithelium. In addition, using this drug reduces mental symptoms of menopausal syndrome. It is used in the premenopausal and menopausal periods, for skin atrophy and signs of genital degeneration, and so on.
Description
Estriol is a metabolite of estradiol and a major estrogen produced in the later stages of pregnancy. In a longitudinal study in healthy pregnant women, total plasma estriol levels increased from <10 ng/ml at 8-
Chemical properties
White or almost white, crystalline powder.
The Uses of Estriol
17b-estradiol metabolite, primary estrogen in urine
The Uses of Estriol
A metabolite of Estradiol. An estrogenic metabolite considerably less potent than the hormone Estradiol
The Uses of Estriol
A metabolite of Estradiol (E888000). An estrogenic metabolite considerably less potent than the hormone Estradiol (E888000).
What are the applications of Application
Estriol is an estradiol metabolite shown to protect against mammary carcinogenisis
Definition
ChEBI: A 3-hydroxy steroid that is estra-1,3,5(10)-trien-3-ol substituted by additional hydroxy groups at positions 16 and 17 (16alpha,17beta-stereoisomer).
Background
A hydroxylated metabolite of estradiol or estrone that has a hydroxyl group at C3-beta, 16-alpha, and 17-beta position. Estriol is a major urinary estrogen. During pregnancy, large amount of estriol is produced by the placenta. Isomers with inversion of the hydroxyl group or groups are called epiestriol. Though estriol is used as part of the primarily North American phenomenon of bioidentical hormone replacement therapy, it is not approved for use by the FDA or Health Canada. It is however available in the United States by prescription filled only by compounding pharmacies. It has also been approved and marketed throughout Europe and Asia for approximately 40 years for the treatment of post-menopausal hot flashes.
Indications
Used as a test to determine the general health of an unborn fetus.
brand name
Theelol (Parke-Davis).
General Description
Estriol, estra-1,3,5(10)-triene-3,16 ,17 -triol, is available for compounding into several differentformulations for use in HRT. It can be used alone or in combinationswith estradiol (Bi-Est) or with estradiol and estrone(Tri-Est).
Hazard
A carcinogen (OSHA).
Pharmacokinetics
Estriol (also oestriol) is one of the three main estrogens produced by the human body. It is only produced in significant amounts during pregnancy as it is made by the placenta. In pregnant women with multiple sclerosis (MS), estriol reduces the disease's symptoms noticeably, according to researchers at UCLA's Geffen Medical School.
Safety Profile
Suspected carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Other experimental reproductive effects. Mutation data reported. A steroid drug for the treatment of menopause. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
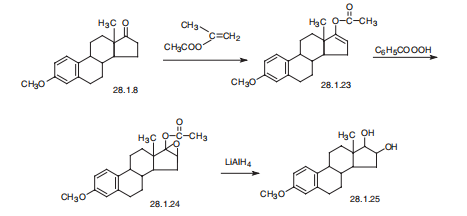
Estriol, estra-1,3,5(10)-trien-3,16|á,17|?-triol (28.1.25), is proposed to be synthesized from the methyl ester of estrone (28.1.8). Methyl ester of estrone is reacted with isopropenylacetate in the presence of p-toluenesulfonic acid, forming the corresponding enolacetate (28.1.23). The resulting enolacetate is oxidized to an epoxide using perbenzoic acid. The resulting epoxide (28.1.24) undergoes reduction by lithium aluminum hydride to.
Metabolism
Purification Methods
Crystallise estriol from EtOH/ethyl acetate. Also purify it by countercurrent distribution with cyclohexane/EtOAc (1:1) and EtOH/H2O (1:1). The UV (EtOH) has max at 280nm ( 2,090 M-1cm-1). [Huffmann & Lott 71 719 1949, Leeds et al. J Am Chem Soc 76 2943 1954, Beilstein 6 IV 7550.]
Properties of Estriol
| Melting point: | 280-282 °C(lit.) |
| Boiling point: | 370.61°C (rough estimate) |
| alpha | D25 +58° ±5° (0.04 g in 1 ml dioxane) |
| Density | 1.27 |
| refractive index | 58 ° (C=0.4, Dioxane) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, sparingly soluble in ethanol (96 per cent). |
| form | neat |
| pka | pKa 10.38±0.02(H2O t=23±2 Iunspeci?ed) (Uncertain) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 3.2mg/L(25 ºC) |
| Merck | 3707 |
| BRN | 2508172 |
| CAS DataBase Reference | 50-27-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Estriol(50-27-1) |
| EPA Substance Registry System | Estriol (50-27-1) |
Safety information for Estriol
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity H362:Reproductive toxicity, effects on or via lactation |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Estriol
| InChIKey | PROQIPRRNZUXQM-ZXXIGWHRSA-N |
Estriol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 50-27-1 Estriol 98%View Details
50-27-1 Estriol 98%View Details
50-27-1 -
 50-27-1 98%View Details
50-27-1 98%View Details
50-27-1 -
 Estriol 99%View Details
Estriol 99%View Details
50-27-1 -
 Estriol, 97% CAS 50-27-1View Details
Estriol, 97% CAS 50-27-1View Details
50-27-1 -
 Estriol 98% CAS 50-27-1View Details
Estriol 98% CAS 50-27-1View Details
50-27-1 -
 Estriol CAS 50-27-1View Details
Estriol CAS 50-27-1View Details
50-27-1 -
 Estriol CAS 50-27-1View Details
Estriol CAS 50-27-1View Details
50-27-1 -
 Estriol Powder APIView Details
Estriol Powder APIView Details
50-27-1
