Eribulin
- CAS NO.:253128-41-5
- Empirical Formula: C40H59NO11
- Molecular Weight: 729.9
- MDL number: MFCD23160037
- EINECS: 803-583-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:03
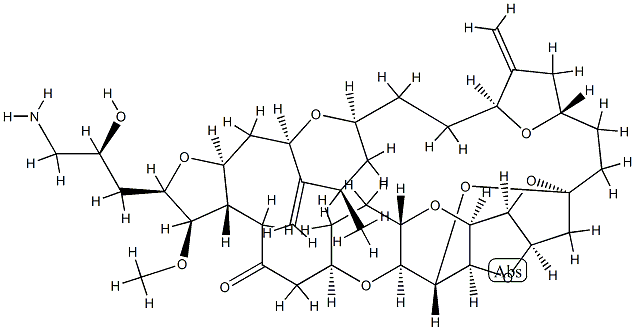
What is Eribulin?
Toxicity
Peripheral neuropathy was the most common toxicity leading to discontinuation of eribulin (5 percent). [Richard Pazdur, M.D., director of the FDA's Division of Oncology Drug Products.] Single doses of 0.75 mg/kg were lethal to rats and two doses of 0.075 mg/kg were lethal to dogs. The no-observed-adverse-effect level (NOAEL) in rats and dogs were 0.015 and 0.0045 mg/kg/day, respectively.
Description
The U.S. FDA approved eribulin mesylate (also referred to as E7389) in November 2010 for the treatment of metastatic breast cancer (MBC) for patients who previously received at least two chemotherapeutic regimens for late-stage disease. Eribulin is a synthetic analog of the marine natural product halichondrin B, which is isolated from the sea sponge Halichondria okadai. Eribulin retains most of the structural elements that constitute the right hand side of halichondrin B; structure–activity relationship (SAR) studies suggested that the antitumor activity of halichondrin B resides in that part of the molecule . Eribulin is a microtubule inhibitor that binds close to the vinca-binding site of tubulin. Unlike most tubulin inhibitors like taxanes, epothilones, and vinca alkaloids that inhibit microtubule dynamic instability by changing tubulin addition and loss parameters, eribulin’s effects on dynamic instability are novel in that eribulin inhibits the growth phase of microtubules without affecting the shortening phase by binding to microtubule plus ends.
Originator
Eisai (United States)
The Uses of Eribulin
Eribulin is a anticancer drug and also is a fully synthetic macrocyclic ketone analogue of the marine natural product halichondrin B. It derived from a marine sponge (Lissodendoryx sp.) with antineoplastic activity.
Indications
For the treatment of patients with metastatic breast cancer who have previously received at least two chemotherapeutic regimens for the treatment of metastatic cancer.
Background
Eribulin is a microtubule inhibitor indicated for the treatment of patients with metastatic breast cancer who have previously received at least two chemotherapeutic regimens for the treatment of metastatic disease. Eribulin was isolated from the marine sponge Halichondria okadai. Eribulin is also being investigated for use in the treatment of advanced solid tumors .
Definition
ChEBI: Eribulin is a fully synthetic macrocyclic ketone analogue of marine sponge natural products. Inhibits growth phase of microtubules via tubulin-based antimitotic mechanism, which leads to G2/M cell-cycle block, disruption of mitotic spindles, and, ultimately, apoptotic cell death after prolonged mitotic blockage It has a role as an antineoplastic agent and a microtubule-destabilising agent. It is a macrocycle, a polyether, a polycyclic ether, a cyclic ketone, a primary amino compound and a cyclic ketal. It is a conjugate base of an eribulin(1+).
brand name
Halaven
Pharmacokinetics
Linear
Clinical Use
Antineoplastic agent:Treatment of metastatic breast cancer
Drug interactions
Potentially hazardous interactions with other drugs Antipsychotics: avoid concomitant use with clozapine (increased risk of agranulocytosis).
Metabolism
There are no major human metabolites of eribulin, CYP3A4 negligibly metabolizes eribulin in vitro.
Metabolism
Minimal metabolism. Eribulin is eliminated primarily by biliary excretion. The transport protein involved in the excretion is presently unknown. Preclinical studies indicate that eribulin is transported by Pgp. However, it is unknown whether Pgp is contributing to the biliary excretion of eribulin.
Mode of action
Eribulin is a non-taxane, microtubule dynamics inhibitor that increases survival of patients with metastatic breast cancer. Eribulin binds to the vinca domain of tubulin and inhibits the polymerization of tubulin and the assembly of microtubules, resulting in inhibition of mitotic spindle assembly, induction of cell cycle arrest at G2/M phase, and, potentially, tumor regression.
Properties of Eribulin
| Density | 1.29±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | 12.56±0.35(Predicted) |
| color | White to off-white |
Safety information for Eribulin
Computed Descriptors for Eribulin
| InChIKey | UFNVPOGXISZXJD-JBQZKEIOSA-N |
Abamectin manufacturer
Related products of tetrahydrofuran
![Methyl 2-((2R,4aS,6S,7R,8S,8aS)-7,8-bis((tert-butyldimethylsilyl)oxy)-6-((S,E)-1-((tert-butyldimethylsilyl)oxy)-3-iodoallyl)octahydropyrano[3,2-b]pyran-2-yl)acetate](https://img.chemicalbook.in/CAS/20211123/GIF/157322-83-3.gif)
![Propanoic acid, 2,2-dimethyl-, 3-[(2S,5S)-tetrahydro-4-methylene-5-[(3R,5R)-5-methyl-3-[(methylsulfonyl)oxy]-6-[[(trifluoromethyl)sulfonyl]oxy]-6-hepten-1-yl]-2-furanyl]propyl ester](https://img.chemicalbook.in/CAS/20180702/GIF/871357-66-3.gif)
![(2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]](https://img.chemicalbook.in/CAS/20200611/GIF/253128-10-8.gif)
![3,6-Anhydro-2,4,7-trideoxy-8,9-bis-O-[(1,1-dimethylethyl)dimethylsilyl]-5-O-methyl-4-[(phenylsulfonyl)methyl]-D-glycero-D-gulo-nonose](https://img.chemicalbook.in/CAS/20210111/GIF/871348-24-2.gif)
You may like
-
 253128-41-5 Eribulin 98%View Details
253128-41-5 Eribulin 98%View Details
253128-41-5 -
 253128-41-5 98%View Details
253128-41-5 98%View Details
253128-41-5 -
 ZIRCONIUM AAS SOLUTION 99%View Details
ZIRCONIUM AAS SOLUTION 99%View Details -
 FUCHSIN BASIC 0.1% SOLUTION 99%View Details
FUCHSIN BASIC 0.1% SOLUTION 99%View Details -
 1 10-PHENANTHROLIN HYDROCHLORIDE 99%View Details
1 10-PHENANTHROLIN HYDROCHLORIDE 99%View Details -
 ERDMANS REAGENT (FOR PROTEIN TEST) 99%View Details
ERDMANS REAGENT (FOR PROTEIN TEST) 99%View Details -
 OXALIC ACID SOLUTION 0.1 N 99%View Details
OXALIC ACID SOLUTION 0.1 N 99%View Details -
 A-AMYLASE (DIASTASE) 99%View Details
A-AMYLASE (DIASTASE) 99%View Details