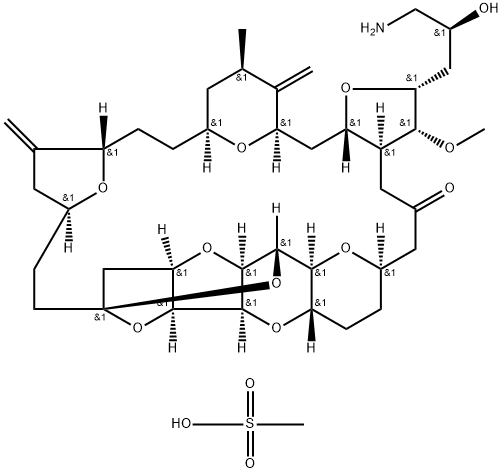
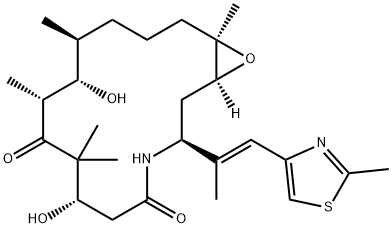
Eribulin Mesylate
- CAS NO.:441045-17-6
- Empirical Formula: C41H63NO14S
- Molecular Weight: 826.01
- MDL number: MFCD22572760
- EINECS: 813-106-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-08 16:17:51
What is Eribulin Mesylate?
The Uses of Eribulin Mesylate
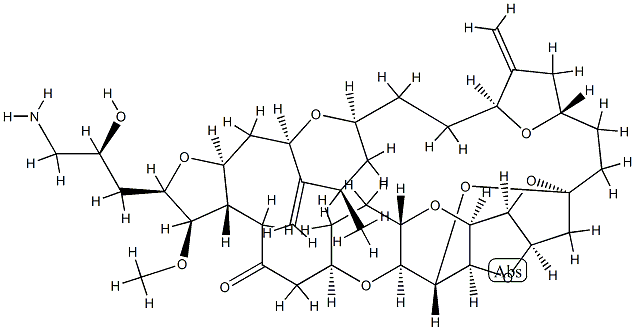
Eribulin Mesylate is a synthetic analog of the marine natural product halichondrin B.
Definition
ChEBI: Eribulin mesylate is a methanesulfonate salt obtained by reaction of eribulin with one equivalent of methanesulfonic acid. A fully synthetic macrocyclic ketone analogue of marine sponge natural products. Inhibits growth phase of microtubules via tubulin-based antimitotic mechanism, which leads to G2/M cell-cycle block, disruption of mitotic spindles, and, ultimately, apoptotic cell death after prolonged mitotic blockage. It has a role as an antineoplastic agent and a microtubule-destabilising agent. It contains an eribulin(1+).
Clinical Use
Eribulin is a highly potent cytotoxic agent approved in the U.S. for the treatment of metastatic breast cancer for patients who have received at least two previous chemotherapeutic regimens. Eribulin was discovered and developed by Eisai and it is currently undergoing clinical evaluation for the treatment of sarcoma (PhIII) and non-small cell lung cancer which shows progression after platinumbased chemotherapy and for the treatment of prostate cancer (PhII). Early stage clinical trials are also underway to evaluate eribulin’s efficacy against a number of additional cancers. Eribulin is a structural analog of the marine natural product halichondrin B. Its mechanism of action involves the disruption of mitotic spindle formation and inhibition of tubulin polymerization which results in the induction of cell cycle blockade in the G2/M phase and apoptosis.
Clinical Use
Eribulin mesylate, a nontaxane, completely synthetic microtubule inhibitor, has recently been approved by the U.S. Food and Drug Administration as third-line treatment of metastatic breast cancer refractory to anthracyclines and taxanes.
Side Effects
Eribulin Mesylate (Halaven) may cause serious side effects including:hives; difficulty breathing; swelling of your face, lips, tongue, or throat; chest pain; severe dizziness; fast or pounding heartbeats, numbness, tingling, or burning pain in your hands or feet; pain or burning when you urinate; confusion; uneven heart rate; extreme thirst; increased urination; leg discomfort; muscle weakness; limp feeling; fever; chill; painful mouth sores; pain when swallowing; cough; trouble breathing; pale skin; easy bruising; sore throat; skin pain followed by a red or purple skin rash that spreads (especially in the face or upper body) and causes blistering and peeling.
Toxicology
Peripheral neuropathy was the most common toxicity leading to discontinuation of eribulin (5 percent). Single doses of 0.75 mg/kg were lethal to rats and two doses of 0.075 mg/kg were lethal to dogs. The no-observed-adverse-effect level (NOAEL) in rats and dogs were 0.015 and 0.0045 mg/kg/day, respectively.
Synthesis
Several synthetic routes for the preparation of
eribulin have been disclosed, each of which utilizes the same strategy described by Kishi and
co-workers for the total synthesis of halichondrin B. Although the scales of these routes were not
disclosed in all cases, this review attempts to highlight what appears to be the production-scale route
based on patent literature. Nonetheless, the synthesis of eribulin represents a significant
accomplishment in the field of total synthesis and brings a novel chemotherapeutic option to cancer
patients.
The strategy to prepare eribulin mesylate (V) employs a convergent synthesis featuring the
following: the late stage coupling of sulfone 22 and aldehylde 23 followed by macrocyclization under
Nozaki-Hiyami-Kishi coupling conditions, formation of a challenging cyclic ketal, and installation of
the primary amine. Sulfone 22 was further simplified to aldehyde 24 and vinyl triflate 25
which were coupled through a Nozaki-Hiyami-Kishi reaction.
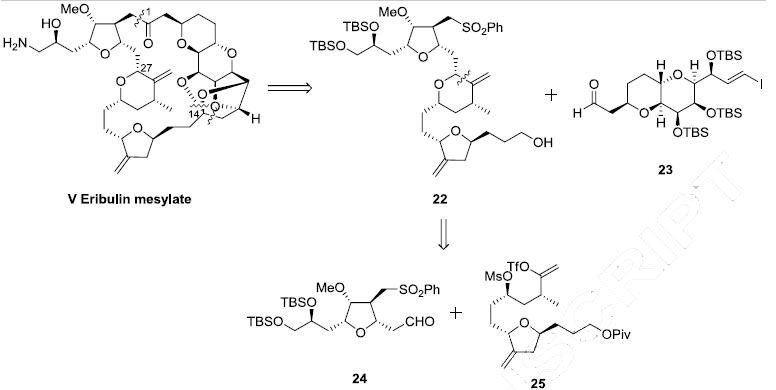
With the three key fragments 23, 24 and 25 along with the entire molecule completed, the next step was to assemble them and complete the
synthesis of eribulin. Aldehyde 24 was coupled to vinyl triflate 25 using an asymmetric Nozaki-
Hiyami-Kishi reaction using CrCl2, NiCl2, Et3N and chiral ligand 67 (the Scheme) to give alcohol 83
(Scheme 11). Formation of the THP ring was accomplished by reaction with KHMDS which allowed
for displacement of the mesylate with the secondary alcohol and provided the THP containing product
in 72% yield for the three steps. The pivalate ester group was removed with DIBAL-H to give the
western fragment 22 in 92% yield.
The completion of the synthesis of eribulin is illustrated in Scheme 12. The lithium anion of sulfone
22 generated upon reaction with nBuLi was coupled to aldehyde 23 to give diol 84 in 84% yield. Both
of the alcohol functional groups of 84 were oxidized using a Dess-Martin oxidation in 90% yield and
the resulting sulfone was removed via a reductive cleavage upon reaction with SmI2 to give ketoaldehyde
85 in 85% yield. Macrocyclization of 85 was accomplished via an asymmetric Nozaki Hiyami-Kishi reaction using CrCl2, NiCl2, Et3N and chiral ligand 67 to give alcohol 86 in 70% yield.
Modified Swern oxidation of the alcohol provided the corresponding ketone in 91% yield and this was
followed by removal of the five silyl ether protecting groups upon reaction with TBAF and subsequent
cyclization to provide ketone 87. Compound 87 was treated with PPTS to provide the ?°caged?± cyclic
ketal 88 in 79% over two steps. The vicinal diol of 88 was reacted with Ts2O in collidine to affect
selective tosylation of the primary alcohol and this crude product was reacted with ammonium
hydroxide to install the primary amine to give eribulin which was treated with methanesulfonic acid in
aqueous ammonium hydroxide to give eribulin mesylate (V) in 84% yield over the final 3 steps.

Mode of action
Eribulin Mesylate is the mesylate salt of a synthetic analogue of halichondrin B, a substance derived from a marine sponge (Lissodendoryx sp.) with antineoplastic activity. Eribulin binds to the vinca domain of tubulin and inhibits the polymerization of tubulin and the assembly of microtubules, resulting in inhibition of mitotic spindle assembly, induction of cell cycle arrest at G2/M phase, and, potentially, tumor regression.
References
Eribulin mesylate: a novel halichondrin B analogue for the treatment of metastatic breast cancer DOI: 10.2146/ajhp110237
From micrograms to grams: scale-up synthesis of eribulin mesylate DOI: 10.1039/c3np70051h
Eribulin mesylate: mechanism of action of a unique microtubule-targeting agent DOI: 10.1158/1078-0432.CCR-14-3252
Properties of Eribulin Mesylate
| storage temp. | Store at -20°C, protect from light, stored under nitrogen |
| solubility | Soluble in DMSO |
| form | Powder |
| color | White to off-white |
Safety information for Eribulin Mesylate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Eribulin Mesylate
| InChIKey | QAMYWGZHLCQOOJ-PEKQNERQNA-N |
Eribulin Mesylate manufacturer
Aspen Biopharma Labs Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![Methyl 2-((2R,4aS,6S,7R,8S,8aS)-7,8-bis((tert-butyldimethylsilyl)oxy)-6-((S,E)-1-((tert-butyldimethylsilyl)oxy)-3-iodoallyl)octahydropyrano[3,2-b]pyran-2-yl)acetate](https://img.chemicalbook.in/CAS/20211123/GIF/157322-83-3.gif)
![Propanoic acid, 2,2-dimethyl-, 3-[(2S,5S)-tetrahydro-4-methylene-5-[(3R,5R)-5-methyl-3-[(methylsulfonyl)oxy]-6-[[(trifluoromethyl)sulfonyl]oxy]-6-hepten-1-yl]-2-furanyl]propyl ester](https://img.chemicalbook.in/CAS/20180702/GIF/871357-66-3.gif)
![(2-Furanpropanol, 5-[2-[(2S,4R,6R)-6-[[(2S,3S,4R,5R)-5-[(2S)-2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]tetrahydro-4-methoxy-3-[(phenylsulfonyl) methyl]-2-furanyl]methyl]tetrahydro-4-methyl-5-methylene-2H-pyran-2-yl]ethyl]](https://img.chemicalbook.in/CAS/20200611/GIF/253128-10-8.gif)
![3,6-Anhydro-2,4,7-trideoxy-8,9-bis-O-[(1,1-dimethylethyl)dimethylsilyl]-5-O-methyl-4-[(phenylsulfonyl)methyl]-D-glycero-D-gulo-nonose](https://img.chemicalbook.in/CAS/20210111/GIF/871348-24-2.gif)
You may like
-
 Eribulin Mesylate 98%View Details
Eribulin Mesylate 98%View Details
441045-17-6 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1