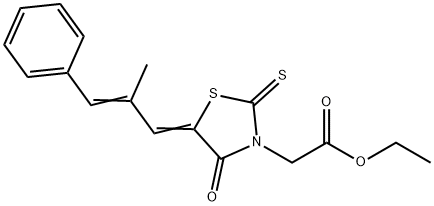
Epalrestat
Synonym(s):(5Z)-5-[(2E)-2-Methyl-3-phenyl-2-propen-1-ylidene]-4-oxo-2-thioxo-3-thiazolidineacetic acid
- CAS NO.:82159-09-9
- Empirical Formula: C15H13NO3S2
- Molecular Weight: 319.4
- MDL number: MFCD00865484
- EINECS: 675-018-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is Epalrestat?
Description
Epalrestat is the second aldose reductase inhibitor to be introduced worldwide and the first to be launched in Japan. The compound is indicated for the treatment of diabetic neuropathy. It is also being investigated for diabetic retinopathy and nephropathy.
Chemical properties
Red Solid
Originator
Ono (Japan)
The Uses of Epalrestat
Epalrestat is an aldose reductase inhibitor with IC50 of 72 nM
The Uses of Epalrestat
An aldose reductase inhibitor. It is used in treatment of diabetic neuropathy.
The Uses of Epalrestat
Epalrestat has been used as an aldose reductase inhibitor:
- in the dahomey larvae diet fed forDrosophila
- for non-irradiated and X-ray irradiated human aldose reductase
- to test its protective effect in mice with bleomycin-induced pulmonary fibrosis
What are the applications of Application
Epalrestat is an aldose reductase inhibitor
Definition
ChEBI: A monocarboxylic acid that is 1,3-thiazolidine which is substituted on the nitrogen by a carboxymethyl group, at positions 2 and 4 by thioxo and oxo groups, respectively, and at position 5 by a 2-methyl-3-phenylprop-2-en-1-ylidene group. It is an inhibitor of aldose reductase (which catalyses the conversion of glucose to sorbitol) and is used for the treatment of some diabetic complications, including neuropathy.
brand name
Kinedak
Biochem/physiol Actions
Epalrestat inhibits Aldose Reductase (AR) involved in the rate limiting step in the conversion of glucose to sorbitol under hyperglycemic conditions. Aldose reductase has been the target of multiple clinical investigatons to treat diabetic neuropathy and retinopathy. Epalrestat is an approved drug in Japan and India, used for the treatment of diabetic neuropathy.
References
1) Kikkawa?et al. (1983)?Effect of a new aldose reductase inhibitor, (E)-3-carboxymethyl-5-[(2E)-methyl-3-phenylpropenylidene]rhodanine (ONO-2235), on peripheral nerve disorders in streptozotocin diabetic rats; Diabetologica,?24?290 2) Terashima?et al. (1984)?Effects of a new aldose reductase inhibitor on various tissues in vitro; J. Pharmacol. Exp. Ther.,?229?226 3) Ramirez and Borja (2008)?Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy; Pharmacotherapy,?28?646
Properties of Epalrestat
| Melting point: | 210-217 C |
| Boiling point: | 516.8±60.0 °C(Predicted) |
| Density | 1.43±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble5mg/mL, clear (warmed) |
| pka | 3.62±0.10(Predicted) |
| form | powder |
| color | yellow to orange |
| Merck | 14,3605 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 2 months. |
| InChI | InChI=1S/C15H13NO3S2/c1-10(7-11-5-3-2-4-6-11)8-12-14(19)16(9-13(17)18)15(20)21-12/h2-8H,9H2,1H3,(H,17,18)/b10-7+,12-8- |
Safety information for Epalrestat
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Epalrestat
| InChIKey | CHNUOJQWGUIOLD-NFZZJPOKSA-N |
| SMILES | S1/C(=C\C(\C)=C\C2=CC=CC=C2)/C(=O)N(CC(O)=O)C1=S |
Epalrestat manufacturer
Related products of tetrahydrofuran

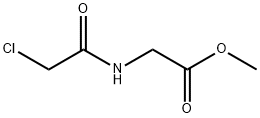
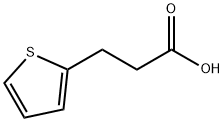
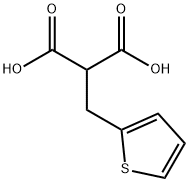
You may like
-
 82159-09-9 Epalrestat 98%View Details
82159-09-9 Epalrestat 98%View Details
82159-09-9 -
 Epalrestat 98%View Details
Epalrestat 98%View Details -
 Epalrestat CAS 82159-09-9View Details
Epalrestat CAS 82159-09-9View Details
82159-09-9 -
 Epalrestat 98%View Details
Epalrestat 98%View Details
82159-09-9 -
 Epalrestat 82159-09-9 98%View Details
Epalrestat 82159-09-9 98%View Details
82159-09-9 -
 82159-09-9 98%View Details
82159-09-9 98%View Details
82159-09-9 -
 Epalrestat 97% CAS 82159-09-9View Details
Epalrestat 97% CAS 82159-09-9View Details
82159-09-9 -
 Epalrestat CAS 82159-09-9View Details
Epalrestat CAS 82159-09-9View Details
82159-09-9