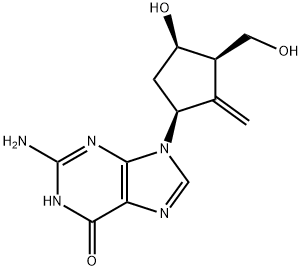
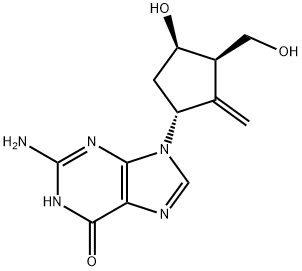
Entecavir
Synonym(s):2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one;2-Amino-9-[(1S,3R,4S)-4-hydroxy-3-hydroxymethyl-2-methylene-cyclopentyl]-3,9-dihydro-purin-6-one;BMS 200475;SQ 34,676
- CAS NO.:142217-69-4
- Empirical Formula: C12H15N5O3
- Molecular Weight: 277.28
- MDL number: MFCD00907887
- EINECS: 604-279-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-18 14:26:18
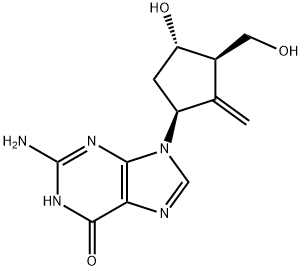
What is Entecavir?
Description
Entecavir is a cyclopentyl guanosine analog launched for the once-daily oral treatment of chronic hepatitis B virus (HBV) infection, and it is the third nucleoside or nucleotide analog to be marketed for this indication. Lamivudine, a deoxythiacytosine analog, and adefovir dipivoxil, a nucleotide analog, have been marketed since 1998 and 2002, respectively. Entecavir and adefovir are specifically indicated for HBV, whereas lamivudine is indicated for both HBV and HIV infections.
Chemical properties
White to Off-White/Yellow Crystalline Powder
Originator
BMS (US)
The Uses of Entecavir
Entecavir is a new generation of guanine nucleoside analogues oral medicine for treatment of hepatitis B virus infection in, mainly for the treatment of adult patients with viral replication activity and serum transaminase continued to increase, or liver tissue for pathological activity of chronic hepatitis B, is currently down virus the fastest and the most powerful, the mutation rate lowest nucleoside analogues.
Indications
For the treatment of chronic hepatitis B virus infection in adults with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease.
Background
Entecavir is an oral antiviral drug used in the treatment of hepatitis B infection. It is marketed under the trade name Baraclude (BMS).
Entecavir is a guanine analogue that inhibits all three steps in the viral replication process, and the manufacturer claims that it is more efficacious than previous agents used to treat hepatitis B (lamivudine and adefovir). It was approved by the U.S. Food and Drug Administration (FDA) in March 2005.
Definition
ChEBI: Guanine substituted at the 9 position by a 4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl group. A synthetic analogue of 2'-deoxyguanosine, it is a nucleoside reverse transcriptase inhibitor with selective antiviral activity against hepatitis B virus Entecavir is phosphorylated intracellularly to the active triphosphate form, which competes with deoxyguanosine triphosphate, the natural substrate of hepatitis B virus reverse transcriptase, inhibiting every stage of the enzyme's activity, although it ha no activity against HIV. It is used for the treatment of chronic hepatitis B.
Trade name
Baraclude (BMS)
Mechanism of action
Entecavir is a nucleoside analog, or more specifically, a deoxyguanosine analogue that belongs to a class of carbocyclic nucleosides and inhibits reverse transcription, DNA replication and transcription in the viral replication process.
Pharmacokinetics
Entecavir is a guanosine nucleoside analogue with selective activity against hepatitis B virus (HBV). It is designed to selectively inhibit the Hepatitis B virus, blocking all three steps in the replication process. Entecavir is more efficient than an older Hepatitis B drug, lamivudine.
Pharmacokinetics
Entecavir had a mean terminal half-life ranging from 128 to 149 hours and an effective half-life of approximately 24 hours. Elimination was predominantly through renal excretion, with mean urinary recovery ranging from 62% to 73%.
Clinical Use
Treatment of chronic hepatitis B virus infection in patients >16 years of age.
Entecavir comes as a tablet and solution (liquid) to take by mouth. It is usually taken once a day on an empty stomach, at least 2 hours after a meal and at least 2 hours before the next meal. Take entecavir at around the same time every day.
Side Effects
The most common side effects of entecavir: the increase of ALT, fatigue, dizziness, nausea, abdominal pain, abdominal discomfort, abdominal discomfort, liver, muscle, insomnia, rubella and indigestion, also be found in neutrophils decreased slightly.
These adverse reactions were mild to moderate. It also found that, as the same type of antiviral drugs, entecavir and the first generation of antiviral drugs have similar side effects, such as acid poisoning, hepatomegaly, liver fatty degeneration in the withdrawal will appear rebound phenomenon.
Synthesis
Entecavir is synthesized from 4-trimethylsilyl-3-butyn-2-one and acrolein. The key features of its preparation are: (1) a stereoselective boron–aldol reaction to afford the acyclic carbon skeleton of the methylenecylopentane moiety; (2) its cyclization by a Cp2TiCl-catalyzed intramolecular radical addition of an epoxide to an alkyne; and (3) the coupling with a purine derivative by a Mitsunobu reaction.
Side Effects
The most common side effects include headache, fatigue, dizziness, and nausea. Less common effects include trouble sleeping and gastrointestinal symptoms such as sour stomach, diarrhea, and vomiting.
Serious side effects from entecavir include lactic acidosis, liver problems, liver enlargement, and fat in the liver.
Absorption
Absorption Following oral administration in healthy subjects, entecavir peak plasma concentrations occurred between 0.5 and 1.5 hours. In healthy subjects, the bioavailability of the tablet is 100% relative to the oral solution.
Metabolism
Entecavir is not a substrate, inhibitor, or inducer of the cytochrome P450 (CYP450) enzyme system. Entecavir is efficiently phosphorylated to the active triphosphate form.
Toxicity
Healthy subjects who received single entecavir doses up to 40 mg or multiple doses up to 20 mg/day for up to 14 days had no increase in or unexpected adverse events. If overdose occurs, the patient must be monitored for evidence of toxicity, and standard supportive treatment applied as necessary.
Storage
Store at -20°C
Properties of Entecavir
| Melting point: | 249-252°C |
| Boiling point: | 661.4±65.0 °C(Predicted) |
| Density | 1.81±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly), Methanol (Sparingly) |
| form | powder |
| pka | 14.22±0.60(Predicted) |
| color | white to beige |
| optical activity | [α]/D +25 to +40°, c = 0.2 in H2O |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 142217-69-4(CAS DataBase Reference) |
Safety information for Entecavir
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H320:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P330:Rinse mouth. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Entecavir
Entecavir manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-(benzyloxy)-3-(benzyloxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one](https://img.chemicalbook.in/CAS/GIF/142217-81-0.gif)
![2-AMino-1,9-dihydro-9-[(1S,3R,4S)-4-(hydroxydiMethylsilyl)-3-(hydroxyMethyl)-2-Methylenecyclopentyl]-6H-purin-6-one](https://img.chemicalbook.in/CAS/GIF/870614-82-7.gif)
You may like
-
 Entecavir 98%View Details
Entecavir 98%View Details
142217-69-4 -
 142217-69-4 Entecavir 99%View Details
142217-69-4 Entecavir 99%View Details
142217-69-4 -
 Entecavir 98%View Details
Entecavir 98%View Details
142217-69-4 -
 142217-69-4 Entecavir 98%View Details
142217-69-4 Entecavir 98%View Details
142217-69-4 -
 Entecavir 99%View Details
Entecavir 99%View Details
142217-69-4 -
 Entecavir 98% CAS 142217-69-4View Details
Entecavir 98% CAS 142217-69-4View Details
142217-69-4 -
 Entecavir CAS 142217-69-4View Details
Entecavir CAS 142217-69-4View Details
142217-69-4 -
 Entecavir 1 mg Entekor 1mg tabletsView Details
Entecavir 1 mg Entekor 1mg tabletsView Details
142217-69-4
