142217-69-4
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | White to off white powder |
| Solubility | Slightly soluble (2.4 mg/mL at pH 7.9, 25 °C) |
| LogP | -0.8 |
| pH | pH = 7.9 in a saturated solution of water at 25 °C |
| Other Experimental Properties | White to off-white powder, mp: >220 °C. Specific optical rotation: +35 deg at 25 °C/D (c = 0.38 in water). Solubility in water: 2.4 mg/mL. pH of saturated solution in water is 7.9 at 25 °C /Entecavir monohydrate/ |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H320:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P330:Rinse mouth. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
COMPUTED DESCRIPTORS
| Molecular Weight | 277.28 g/mol |
|---|---|
| XLogP3 | -1.3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 277.11748936 g/mol |
| Monoisotopic Mass | 277.11748936 g/mol |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 480 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
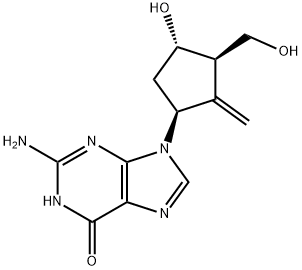
Entecavir (anhydrous) is guanine substituted at the 9 position by a 4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl group. A synthetic analogue of 2'-deoxyguanosine, it is a nucleoside reverse transcriptase inhibitor with selective antiviral activity against hepatitis B virus. Entecavir is phosphorylated intracellularly to the active triphosphate form, which competes with deoxyguanosine triphosphate, the natural substrate of hepatitis B virus reverse transcriptase, inhibiting every stage of the enzyme's activity, although it has no activity against HIV. It is used for the treatment of chronic hepatitis B. It has a role as an EC 2.7.7.49 (RNA-directed DNA polymerase) inhibitor and an antiviral drug. It is a member of 2-aminopurines, an oxopurine, a primary alcohol and a secondary alcohol.
