alpha-Lobeline hydrochloride
Synonym(s):α-Lobeline hydrochloride;(−)-Lobeline hydrochloride;L -Lobeline hydrochloride
- CAS NO.:134-63-4
- Empirical Formula: C22H28ClNO2
- Molecular Weight: 373.92
- MDL number: MFCD00082455
- EINECS: 205-150-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
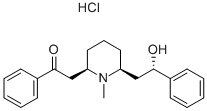
What is alpha-Lobeline hydrochloride?
Description
(–)-Lobeline is an alkaloid that has been found in Lobelia and has diverse biological activities. It binds to nicotinic acetylcholine receptors (nAChRs) in rat brain homogenates (Ki = 4 nM) and has antinociceptive effects in the tail-flick assay in mice. (–)-Lobeline (0.3 and 0.9 mg/kg) reduces the number of errors in a repeated acquisition procedure in the radial arm maze in rats. It also decreases immobility time in the forced swim test and feeding latency in the novelty suppressed feeding test, indicating antidepressant-like activity, in mice when administered at doses of 1 and 4 mg/kg.
Chemical properties
White to Off-White Solid
The Uses of alpha-Lobeline hydrochloride
A neuronal nicotinic acetylcholine receptor agonist. A respiratory stimulant
The Uses of alpha-Lobeline hydrochloride
A neuronal nicotinic acetylcholine receptor agonist. A CNS stimulant.
What are the applications of Application
Lobeline hydrochloride is an AChR agonist
Biological Activity
High affinity nicotinic receptor ligand, with a K i value of 4.4 nM in rat brain.
in vitro
clastogenicity of lobeline and possible interactions between lobeline and ethyl alcohol were investigated in a mutagen-sensitivity assay on cultures of human lymphoblastoid cell lines. lobeline alone was not clastogenic, but there was a marked increase in genetic damage resulting from a coclastogenic interaction between lobeline and ethyl alcohol. [5]
in vivo
male c57bl/6j mice were individually housed and acclimatized to 10% alcohol. lobeline is a partial nicotinic agonist that attenuates alcohol consumption and preference in male c57bl/6j mice. [3] cf-1 male mice received an intraperitoneal injection of lobeline (5 or 10mg/kg). lobeline did not show genotoxic or mutagenic effects and did not increase the ethanol-induced genotoxic effects in blood. lobeline also protected blood cells against oxidative damage induced by hydrogen peroxide. [4]
Properties of alpha-Lobeline hydrochloride
| Melting point: | 183-185 °C (dec.)(lit.) |
| alpha | D20 -43° (c = 2) |
| refractive index | -57.5 ° (C=1, H2O) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 25 mg/mL |
| form | powder |
| color | white to off-white |
| optical activity | [α]20/D 56.8°, c = 2 in H2O |
| Merck | 14,5551 |
| BRN | 3920196 |
| CAS DataBase Reference | 134-63-4(CAS DataBase Reference) |
Safety information for alpha-Lobeline hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
Computed Descriptors for alpha-Lobeline hydrochloride
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 (−)-Lobeline hydrochloride CAS 134-63-4View Details
(−)-Lobeline hydrochloride CAS 134-63-4View Details
134-63-4 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1