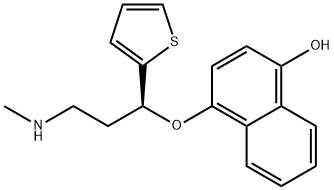
Duloxetine
- CAS NO.:116539-59-4
- Empirical Formula: C18H19NOS
- Molecular Weight: 297.41
- MDL number: MFCD06801358
- EINECS: 601-438-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
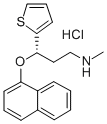
What is Duloxetine?
Absorption
Duloxetine is incompletely absorbed with a mean bioavailability of 50% although there is wide variability in the range of 30-80%. The population absorption constant (ka) is 0.168 h-1.The molecule is susceptible to hydrolysis in acidic environments necessitating the use of an enteric coating to protect it during transit through the stomach. This creates a 2 hour lag time from administration to the start of absorption. The Tmax is 6 hours including the lag time. Administering duloxetine with food 3 hour delay in Tmax along with an 10% decrease in AUC. Similarly, administering the dose at bedtime produces a 4 hour delay and 18% decrease in AUC with a 29% reduction in Cmax. These are attributed to delayed gastric emptying in both cases but are not expected to impact therapy to a clinically significant degree.
Toxicity
Overdose
Fatalities have been reported with doses of 1000mg involving both mixed drugs as well as duloxetine alone. Signs and symptoms of overdose include: somnolence, coma, serotonin syndrome, seizure, syncope, hypo- or hypertension, tachycardia, and vomiting. No antidote exists and the drug is unlikely to be cleared by hemodialysis. Supportive care is recommended along with activated charcoal and gastric lavage to reduce absorption. If serotonin syndrome occurs specific treatment such as temperature control or cyproheptadine may be initiated.
Carcinogenicity & Mutagenicity
Increased incidence of hepatocellular carcinomas and adenomas were reported in female mice fed 140 mg/kg/day duloxetine for 2 years, equivalent to 6 times the maximum recommended human dose (MRHD). No effect was reported with doses of 50mg/kg/day (2 time MRHD) in females or 100 mg/kg/day in males (4 times MRHD). Similar investigation in rats produced no carcinogenicity at doses of 27 mg/kg/day (2 times MRHD)in females and 36 mg/kg/day in males (4 times MRHD).
No mutagenicity, clastogenicity, induction of sister chromatid exchange, or genotoxicity has been observed in toxicology investigations.
Reproductive Toxicity
Neither male or female rats displayed adverse reproductive effects at doses up to 45 mg/kg/day (4 times MRHD).
Lactation
An estimated 25% of plasma duloxetine appears in breast milk with the estimated daily infant dose being 0.14% of the maternal dose. Breast milk concentrations have been observed to peak 3 hours after administration.
The Uses of Duloxetine
Antidepressant.
Indications
Indicated for:
1) Management of Major Depressive Disorder.
2) Management of Generalized Anxiety Disorder.
3) Management of diabetic peripheral neuropathy.
4) Management of fibromyalgia.
5) Management of chronic musculoskeletal pain.
6) Management of osteoarthritis of the knee in adults.
7) Management of chronic lower back pain in adults.
8) Management of stress urinary incontinence in adult women.
Off-label uses include:
1) Management of chemotherapy-induced peripheral neuropathy.
2) Management of stress urinary incontinence in adult men after prostatectomy until recovery is complete.
What are the applications of Application
Duloxetine is an inhibitor of ST and SLC6A2
Background
Duloxetine is a dual serotonin and norepinephrine reuptake inhibitor. It was originally discovered in 1993 and developed by Eli Lilly and Company as LY248686. Duloxetine first received approval from the FDA in August, 2004 as Cymbalta for the treatment of Major Depressive Disorder. It has since received approval for a variety of indications including the treatment of neuropathic pain, Generalized Anxiety disorder, osteoarthritis, and stress incontinence. Duloxetine continues to be investigated for the treatment of pain in cancer, surgery, and more.
Definition
ChEBI: (S)-duloxetine is a duloxetine. It is an enantiomer of a (R)-duloxetine.
brand name
Cymbalta (Lilly).
General Description
Duloxetine (Cymbalta) is a newer antidepressant. It islargely like venlafaxine, which is an SNERI (selective norepinephrinereuptake inhibitor).
Pharmacokinetics
Duloxetine, through increasing serotonin and norepinephrine concentrations in Onuf's nucleus, enhances glutamatergic activation of the pudendal motor nerve which innervates the external urethral sphinter. This enhanced signaling allows for stronger contraction. Increased contraction of this sphincter increases the pressure needed to produce an incontinence episode in stress urinary incontinence. Duloxetine has been shown to improve Patient Global Impression of Improvement and Incontinence Quality of Life scores. It has also been shown to reduce the median incontinence episode frequency at doses of 40 and 80 mg.
Action at the dorsal horn of the spinal cord allows duloxetine to strengthen the the serotonergic and adrenergic pathways involved in descending inhibition of pain. This results in an increased threshold of activation necessary to transmit painful stimuli to the brain and effective relief of pain, particularly in neuropathic pain. Pain relief has been noted in a variety of painful conditions including diabetic peripheral neuropathy, fibromyalgia, and osteoarthritis using a range of pain assessment surveys.
While duloxetine has been shown to be effective in both animal models of mood disorders and in clinical trials for the treatment of these disorders in humans, the broad scope of its pharmacodynamic effects on mood regulation in the brain has yet to be explained.
Increased blood pressure is a common side effect with duloxetine due to vasoconstriction mediated by the intended increase in norepinephrine signaling.
Pharmacokinetics
Duloxetine appears to be fairly well absorbed after oral doses, with peak plasma levels in 6 to 10 hours and
linear pharmacokinetics. The drug is extensively metabolized in the liver to active
metabolites, with 72% of an oral dose primarily excreted in the urine as conjugated metabolites and up to
15% appearing in the feces.
N-demethylation to an active metabolite (CYP2D6) and hydroxylation of the naphthyl ring (CYP1A2) at either
the 4-, 5-, or 6-positions are the main metabolic pathways for duloxetine. Its metabolites are primarily
excreted into the urine as glucuronide, sulfate, and O-methylated conjugation products. The
major metabolites found in plasma also were found in the urine. Preclinical data for 4-hydroxyduloxetine
suggests it has a similar pharmacological profile to duloxetine, with selective inhibition of SERT but less
activity at the NET.
Clinical Use
Duloxetine has been approved for the treatment of depression and diabetic peripheral neuropathic pain. It is another analogue in the line of fluoxetine-based products from Lilly, in which the phenyl and phenoxy groups of fluoxetine have been respectively replaced with the benzene isostere, thiophene, and a naphthyloxy group (previously described under fluoxetine). Duloxetine exhibits dual inhibition with high affinity for the SERTs and NETs, with a five times preferential inhibition of the SERT. Duloxetine appears to be a more potent in vitro blocker of SERTs and NETs than venlafaxine. In humans, duloxetine has a low affinity for the other neuroreceptors, suggesting low incidence of unwanted adverse effects.
Synthesis
Reaction of 2-acetylthiophene
with paraformaldehyde and dimethylamine in
ethanol gives 3-(dimethylamino)-1-(2-thienyl)-
1-propanone, which is enantioselectively reduced
with a 2:1 complex of (2R,3S)-4-(dimethylamino)-
3-methyl-1,2-diphenyl-2-butanol
and LiAlH4 in toluene to yield (S)-3-(dimethylamino)-
1-(2-thienyl)-1-propanol. The
condensation of (S)-3-(dimethylamino)-1-(2-
thienyl)-1-propanol with 1-fluoronaphthalene
catalyzed by NaH in DMSO affords the corresponding
naphthyl ether (S)-N,N-dimethyl-3-
(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-
1-amine, which is finally monodemethylated
with 2,2,2-trichloroethyl chloroformate and zinc
in toluene and treated with oxalic acid .
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: metabolism inhibited by ciprofloxacin
- avoid.
Anticoagulants: possibly increased risk of bleeding
with dabigatran.
Other CNS medication: enhanced effect.
Antidepressants: avoid with MAOIs, moclobemide,
St John’s wort, tryptophan, venlaflaxine, amitriptyline,
clomipramine and SSRIs due to increased risk of
serotonin syndrome; increased risk of side effects
with tricyclic antidepressants; fluvoxamine decreases
the clearance of duloxetine by 77% - avoid; possible
increased risk of convulsions with vortioxetine.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Dapoxetine: avoid concomitant use.
Methylthioninium: risk of CNS toxicity - avoid if
possible.
Metabolism
Duloxetine is extensively metabolized primarily by CYP1A2 and CYP2D6 with the former being the greater contributor. It is hydroxylated at the 4, 5, or 6 positions on the naphthalene ring with the 4-hydroxy metabolite proceeding directly to a glucuronide conjugate while the 5 and 6-hydroxy metabolites proceed through a catechol and a 5-hydroxy, 6-methoxy intermediate before undergoing glucuronide or sulfate conjugation. CYP2C9 is known to be a minor contributor to the 5-hydroxy metabolite. Another uncharacterized metabolite is known to be excreted in the feces but comprises <5% of the total excreted drug. Many other metabolites exist but have not been identified due their low contribution to the overall profile of duloxetine and lack of clinical significance.
Metabolism
Duloxetine is extensively metabolised and the metabolites are excreted principally in urine. Both cytochromes P450-2D6 and 1A2 catalyse the formation of the two major metabolites, glucuronide conjugate of 4-hydroxy duloxetine and sulphate conjugate of 5-hydroxy, 6-methoxy duloxetine. Based upon in vitro studies, the circulating metabolites of duloxetine are considered pharmacologically inactive
Properties of Duloxetine
| Boiling point: | 466.2±40.0 °C(Predicted) |
| Density | 1.158±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | 10.02±0.10(Predicted) |
| InChI | InChI=1/C18H19NOS.ClH/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16;/h2-10,13,17,19H,11-12H2,1H3;1H/t17-;/s3 |
| EPA Substance Registry System | 2-Thiophenepropanamine, N-methyl-?-(1-naphthalenyloxy)-, (?S)- (116539-59-4) |
Safety information for Duloxetine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P501:Dispose of contents/container to..… |
Computed Descriptors for Duloxetine
| InChIKey | BFFSMCNJSOPUAY-VOPAOICTNA-N |
| SMILES | C1(=CC=CS1)[C@H](CCNC)OC1=CC=CC2=CC=CC=C12.Cl |&1:5,r| |
Duloxetine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 116539-59-4 Duloxetine 98%View Details
116539-59-4 Duloxetine 98%View Details
116539-59-4 -
 116539-59-4 Duloxetine 98%View Details
116539-59-4 Duloxetine 98%View Details
116539-59-4 -
 116539-59-4 98%View Details
116539-59-4 98%View Details
116539-59-4 -
 Duloxetine 95% CAS 116539-59-4View Details
Duloxetine 95% CAS 116539-59-4View Details
116539-59-4 -
 Duloxetine Intermediate PowderView Details
Duloxetine Intermediate PowderView Details
116539-59-4 -
 Duloxetine API PowderView Details
Duloxetine API PowderView Details
116539-59-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
