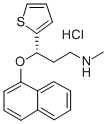
Duloxetine hydrochloride
Synonym(s):(S)-Duloxetine hydrochloride;Duloxetine hydrochloride solution;(γS)-N-Methyl-γ-(1-naphthalenyloxy)-2-thiophenepropanamine hydrochloride;(+)-(S)-N-Methyl-γ-(1-naphthyloxy)-2-thiophenepropylamine hydrochloride;(+)-N-Methyl-3-(1-naphthalenyloxy)-3-(2-thienyl)propanamine hydrochloride
- CAS NO.:136434-34-9
- Empirical Formula: C18H20ClNOS
- Molecular Weight: 333.88
- MDL number: MFCD06407958
- EINECS: 603-962-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-04 20:40:58
What is Duloxetine hydrochloride?
Description
Duloxetine is a selective serotonin (5-HT) and norepinephrine reuptake inhibitor (SSNRI) for oral administration, and it is currently approved in the US and in Europe for the treatment of major depressive disorder (MDD) and diabetic peripheral neuropathic pain (DPN). Additionally, it is indicated for the treatment of stress urinary incontinence (SUI) in Europe.
Chemical properties
White Crystalline Solid
Originator
Lilly (US)
The Uses of Duloxetine hydrochloride
Duloxetine Hydrochloride is an antidepressant that functions as a dual serotonin and norepinephrine reuptake inhibitor (SNRI). It exhibits linear pharmacokinetics in mice and is not associated with any drastic changes in blood pressure or heart rate.
The Uses of Duloxetine hydrochloride
Labelled Duloxetine, which is used as an antidepressant. A dual serotonin and norepinephrine reuptake inhibitor (SNRI). Current lot is 97% d7 with no d0, d1 or d2
The Uses of Duloxetine hydrochloride
An antidepressant. A dual serotonin and norepinephrine reuptake inhibitor (SNRI). Used in treatment of stress urinary incontinence.
The Uses of Duloxetine hydrochloride
leucotriene antagonist, antiasthmatic
The Uses of Duloxetine hydrochloride
anti-depressant, analgesic for osteoarthiritis and musculoskeletal pain
The Uses of Duloxetine hydrochloride
solubility H2O: soluble5 mg/mL (clear solution, warmed)
Definition
ChEBI: A duloxetine hydrochloride in which the duloxetine moiety has S configuration.
What are the applications of Application
Duloxetine Hydrochloride is a dual serotonin and norepinephrine reuptake inhibitor.
brand name
Cymbalta (Lilly).
Biochem/physiol Actions
Duloxetine hydrochloride is a dual serotonin/norepinephrine reuptake inhibitor (SNRI), widely used clinically as an antidepressant and anxiolytic.
Synthesis
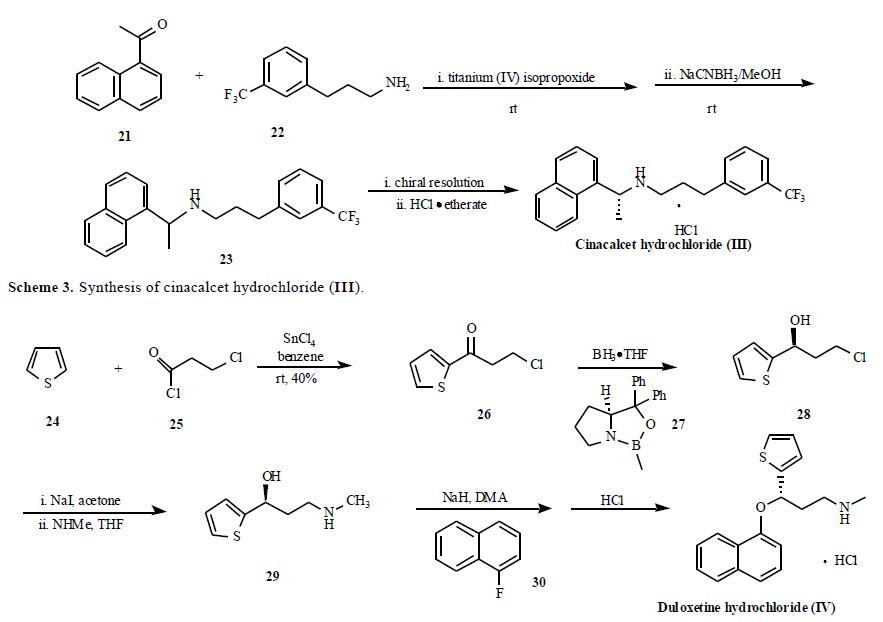
The synthesis from Lilly?ˉs group is depicted in Scheme 4. Friedel-Crafts acylation of thiophene (24) by 3- chloropropanoyl chloride (25) with SnCl4 as Lewis acid gave ketone 26 which was then enantioselectively reduced with (R )-1-methyl-3,3-diphenyl-tetrahydropyrrolo[1,2- c][1,3,2]oxazaborole (27) in the presence of borane in THF to give (S)-3-chloro-1-(2-thienyl)-1-propanol (28). Compound 28 was subjected to Finkelstein reaction to give (S)-3-iodio-1-(2-thienyl)-1-propanol which was reacted with methylamine in THF to give compound 29. The alcohol 29 was then used in a nucleophilic displacement reaction with 1-fluoronaphthalene (30) in the presence of sodium hydride in DMA to give duloxetine free base in 88% yield. Finally, the free base was treated with HCl to yield duloxetine hydrochloride (IV).
storage
Room temperature
Properties of Duloxetine hydrochloride
| Melting point: | 118-122°C |
| Flash point: | 9℃ |
| storage temp. | room temp |
| solubility | H2O: soluble5mg/mL (clear solution, warmed) |
| form | powder |
| pka | pKa in DMF-water (66:34): 9.6(at 25℃) |
| color | white to beige |
| optical activity | [α]/D +116 to +125°, c = 1 in methanol |
| Merck | 14,3465 |
| InChI | InChI=1/C18H19NOS.ClH/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16;/h2-10,13,17,19H,11-12H2,1H3;1H/t17-;/s3 |
Safety information for Duloxetine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Duloxetine hydrochloride
| InChIKey | BFFSMCNJSOPUAY-VOPAOICTNA-N |
| SMILES | C1(=CC=CS1)[C@H](CCNC)OC1=CC=CC2=CC=CC=C12.Cl |&1:5,r| |
Duloxetine hydrochloride manufacturer
SOLISOM HEALTHCARE LLP
Styrax Pharma Pvt Ltd
Chemeca Drugs Private Limited (Vegesna Laboratories Pvt Ltd)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



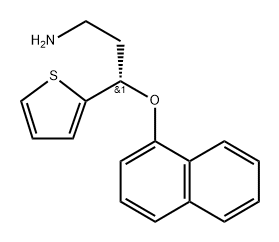
You may like
-
 136434-34-9 DULOXETINE HCL 98%View Details
136434-34-9 DULOXETINE HCL 98%View Details
136434-34-9 -
 136434-34-9 98%View Details
136434-34-9 98%View Details
136434-34-9 -
 Duloxetine hydrochloride 136434-34-9 98%View Details
Duloxetine hydrochloride 136434-34-9 98%View Details
136434-34-9 -
 Duloxetine hydrochloride 98%View Details
Duloxetine hydrochloride 98%View Details
136434-34-9 -
 Duloxetine hydrochloride 98%View Details
Duloxetine hydrochloride 98%View Details -
 Duloxetine hydrochloride 98%View Details
Duloxetine hydrochloride 98%View Details -
 Duloxetine Hydrochloride CAS 136434-34-9View Details
Duloxetine Hydrochloride CAS 136434-34-9View Details
136434-34-9 -
 Duloxetine hydrochloride 136434-34-9 99%View Details
Duloxetine hydrochloride 136434-34-9 99%View Details
136434-34-9