Droxinostat
Synonym(s):4-(4-Chloro-2-methylphenoxy)-N-hydroxybutanamide
- CAS NO.:99873-43-5
- Empirical Formula: C11H14ClNO3
- Molecular Weight: 243.69
- MDL number: MFCD01326592
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-14 14:09:35
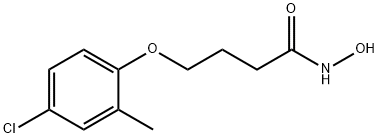
What is Droxinostat?
The Uses of Droxinostat
Droxinostat is a histone deacetylase selective inhibitor and is used in cancer therapy and cancer research. Droxinostat is a selective inhibitor of HDAC3, HDAC6, and HDAC8.
What are the applications of Application
Droxinostat is a selective inhibitor of HDAC3, HDAC6, and HDAC8
Definition
ChEBI: 4-(4-chloro-2-methylphenoxy)-N-hydroxybutanamide is an aromatic ether.
Biological Activity
droxinostat is a selective inhibitor of hdac3, hdac6, and hdac8 with ic50 value of 16.9 ± 5.0 μm, 2.47 ± 1.09 μm, and 1.46 ± 0.11 μm, respectively [1].hdacs (histone deacetylases) are enzymes responsible of the deacetylation of lysine that residues of core histones and play a pivotal role in controlling chromatin remodeling and transcriptional activation. it is also reported that hdacs control the acetylation and activation status of multiple non-histone proteins, including the heat shock protein 90 (hsp90) which is an essential molecular chaperone for fungal virulence and antifungal resistance. multiple hdacs have been identified and hdac1 to hdac10 are shown to express in malignant cells which reminds the hdac inhibitor as a target for cancer therapy [2] [3].droxinostat is a selective hdac inhibitor and is different from the known pan-hdaci tsa which inhibits all tested hdac. when tested with prostate cancer line ppc-1 cells, droxinostat treatment selectively inhibited hdac3, hdac6, and hdac8 activity at the concentration of 50 μm/l which sensitized cells to death ligands [1]. in androgen-dependent cap cells, administration of droxinostat selectively inhibited hdacs and downregulated c-flp expression which resulted in cells apoptosis [4].
References
[1]. wood, t.e., et al., selective inhibition of histone deacetylases sensitizes malignant cells to death receptor ligands. mol cancer ther, 2010. 9(1): p. 246-56.
[2]. lamoth, f., p.r. juvvadi, and w.j. steinbach, histone deacetylase inhibition as an alternative strategy against invasive aspergillosis. front microbiol, 2015. 6: p. 96.
[3]. mackmull, m.t., et al., histone deacetylase inhibitors cause the selective depletion of bromodomain containing proteins. mol cell proteomics, 2015.
[4]. mccourt, c., et al., elevation of c-flip in castrate-resistant prostate cancer antagonizes therapeutic response to androgen receptor-targeted therapy. clin cancer res, 2012. 18(14): p. 3822-33.
Properties of Droxinostat
| Density | 1.252 |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO: ≥20mg/mL |
| form | powder |
| color | white to off-white |
Safety information for Droxinostat
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Droxinostat
New Products
(R)-3-Aminobutanenitrile Hydrochloride 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION SODIUM METHYL PARABEN Methylcobalamin (vitamin B12) Diclofenac Sodium SODIUM VALPROATE Racecadotril XANTHAN GUM 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 Droxinostat 98.00% CAS 99873-43-5View Details
Droxinostat 98.00% CAS 99873-43-5View Details
99873-43-5 -
 3-Bromo-2-hydroxybenzeneacetic acid 98%View Details
3-Bromo-2-hydroxybenzeneacetic acid 98%View Details
88491-46-7 -
 1805639-70-6 98%View Details
1805639-70-6 98%View Details
1805639-70-6 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 25408-95-1 98%View Details
25408-95-1 98%View Details
25408-95-1 -
 1-(3-Chloro-2-hydroxyphenyl)-2-propanone 98%View Details
1-(3-Chloro-2-hydroxyphenyl)-2-propanone 98%View Details
1784294-80-9 -
 99903-60-3 98%View Details
99903-60-3 98%View Details
99903-60-3 -
 5-Fluoro-2-iodo-3-methylaniline 1823368-42-8 98%View Details
5-Fluoro-2-iodo-3-methylaniline 1823368-42-8 98%View Details
1823368-42-8