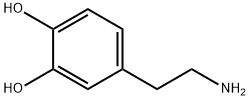
Dopamine hydrochloride
Synonym(s):2-(3,4-Dihydroxyphenyl)ethylamine hydrochloride;3,4-Dihydroxyphenethylamine hydrochloride;3-Hydroxytyramine hydrochloride;4-(2-Aminoethyl)-1,2-benzenediol hydrochloride
- CAS NO.:62-31-7
- Empirical Formula: C8H12ClNO2
- Molecular Weight: 189.64
- MDL number: MFCD00012898
- EINECS: 200-527-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
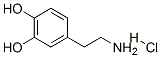
What is Dopamine hydrochloride?
Description
Dopamine (hydrochloride) is an endogenous catecholamine neurotransmitter synthesized from the amino acid L-tyrosine that acts as an agonist at dopamine receptors (D1-5). Dopamine is mainly synthesized in the substantia nigra and ventral tegmental area, and is a precursor in norepinephrine and epinephrine biosynthesis. Dopamine-containing neurons in the brain are involved in reward-motivated behavior, motor control, and hormone release. Dopamine is also synthesized in the adrenal glands where it exerts peripheral paracrine functions including control of vasodilation, sodium excretion, insulin production, gastrointestinal motility, and the activity of lymphocytes. Loss or damage of dopaminergic neurons in the substantia nigra is associated with Parkinson’s disease.
Chemical properties
Dopamine hydrochloride is designated chemically as 3,4-dihydroxyphenethylamine hydrochloride, a white crystalline powder freely soluble in water. It can also be dissolved in hot 95% ethanol, methanol, and sodium hydroxide solution. However, it is insoluble in chloroform, ether, and benzene. It has no odor and a slightly bitter taste. Dopamine (also referred to as 3-hydroxytyramine) is a naturally occurring biochemical catecholamine precursor of norepinephrine.
The Uses of Dopamine hydrochloride
Endogenous catecholamine with α and β-adrenergic activity. Cardiotonic; antihypotensive.
The Uses of Dopamine hydrochloride
dopaminergic: Dopamine (DA) works as neurotransmitter in the central nervous system. It is a catecholamine made from the amino acid L-tyrosine. It also works as a hormone in vesicles of the adrenal medulla, thereby controlling heart beat rate and blood pressure. Absence of DA-containing neurons is associated with parkinson′s disease.
The Uses of Dopamine hydrochloride
A neurotransmitter and vasopressor that modulates cortical activation. Dopamine hydrochloride is a vasopressor that moderates cortical activation. It is used as a precursor to norepinepherine and epinephrine. It is an important neurotransmitter and chemical messenger that helps in the transmission of signals in the brain and other vital areas.
What are the applications of Application
3-Hydroxytyramine, Hydrochloride is a major catecholamine neurotransmitter
Indications
Dopamine HCl is indicated for the correction of hemodynamic imbalances present in the shock syndrome due to myocardial infarction, trauma, endotoxic septicemia, open-heart surgery, renal failure, and chronic cardiac decompensation as in congestive failure.
What are the applications of Application
Dopamine hydrochloride has been used to study dopamine-mediated transient modulation of the physiological responses in whiteleg shrimp, Litopenaeus vannamei.
It has been used to study dopamine-mediated changes in immunity susceptibility to Lactococcus garvieae in the freshwater giant prawn, Macrobrachium rosenbergii.
It has been used to study the molecular link between dopamine-induced oxidative stress and mHtt (mutant Huntingtin) toxicity in relation to the activation of the autophagy pathway in an 'in vitro' model of parkinsonian Huntington's Disease.
Definition
ChEBI: Dopamine hydrochloride is a catecholamine.
Preparation
Dopamine hydrochloride is produced through the ring-opening reaction of piperonyl ethylamine. Piperethylamine and phenol are added to a reaction vessel and cooled down. Hydrochloric acid is slowly added while heating up the mixture to 110°C, and refluxed for 12-44 hours until the reaction reaches completion. The solution is then slightly cooled and water is added, and the mixture is stirred well and allowed to separate into layers. The upper phenolic layer is then removed, and the aqueous layer is extracted with isopropyl acetate. The extracted aqueous layer is then evaporated under reduced pressure (8.0kPa) until dry. Half the amount of ethanol and hydrochloric acid are added to the dry residue and heated to dissolve. The solution is then cooled, crystallized, and filtered. The filter cake is washed with ethanol and dried to obtain the final product, with a yield of 74%.
brand name
Intropin (Mayne).
General Description
Dopamine Hydrochloride is the hydrochloride salt form of dopamine, a monoamine compound with positive inotropic activity. Dopamine is a neurotransmitter which is a naturally occurring catecholamine. Dopamine hydrochloride salt is indicated as a medicine for the treatment of acute congestive and renal failures.
Biological Activity
Endogenous neurotransmitter that acts as an agonist at dopamine D 1-5 receptors. Synthesized in the substantia nigra and ventral tegmental area, and is a precursor in noradrenalin and adrenalin biosynthesis.
Pharmacokinetics
Dopamine hydrochloride(62-31-7) is a precursor for the synthesis of epinephrine in the body. It has β (mainly β1 receptor) receptor agonism and α receptor agonism, and can also promote the release of norepinephrine. It can enhance myocardial contractility, increase cardiac output, and accelerate the heart rate to a lesser extent (not as obvious as isoproterenol); stimulate the α-receptors of blood vessels in tissues such as skin and muscles, so that blood vessels constrict and blood supply is reduced; it stimulates visceral blood vessels ( The dopamine receptors in the kidney, mesentery, and heart) dilate and increase blood flow. The change of total peripheral resistance is not obvious, but it is beneficial to improve the blood supply of vital organs during shock.
Clinical Use
Cardiogenic shock in infarction or cardiac surgery
Drug interactions
Potentially hazardous interactions with other drugs
Alpha-blockers: avoid with tolazoline.
Anaesthetics: risk of ventricular arrhythmias with
isoflurane - avoid.
Antidepressants: risk of hypertensive crisis with
MAOIs and moclobemide.
Ciclosporin: may reduce risk of ciclosporin
nephrotoxicity
Dopaminergics: effects possibly enhanced
by entacapone; avoid with rasagiline; risk of
hypertensive crisis with selegiline.
Metabolism
Dopamine is a metabolic precursor of noradrenaline and, whereas a proportion is excreted as the metabolic products of noradrenaline, the majority is mainly metabolised into 3,4,-dihydroxyphenylacetic acid (DOPAC) and 3-methoxy-4-hydroxyphenylacetic (HVA) which are rapidly excreted in the urine.
Storage
Store at -20°C
Properties of Dopamine hydrochloride
| Melting point: | 248-250 °C(lit.) |
| Density | 1.4 g/cm3 |
| Flash point: | 11℃ |
| storage temp. | Store below +30°C. |
| solubility | alcohol: 20 mg/mL |
| form | Crystalline Powder |
| color | light tan |
| Water Solubility | soluble |
| Sensitive | Air & Light Sensitive |
| Merck | 14,8462 |
| BRN | 3656720 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Combustible. |
| CAS DataBase Reference | 62-31-7(CAS DataBase Reference) |
| EPA Substance Registry System | 1,2-Benzenediol, 4-(2-aminoethyl)-, hydrochloride (62-31-7) |
Safety information for Dopamine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Dopamine hydrochloride
| InChIKey | CTENFNNZBMHDDG-UHFFFAOYSA-N |
Dopamine hydrochloride manufacturer
Innovative
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Dopamine hydrochloride 98%View Details
Dopamine hydrochloride 98%View Details -
 1477-71-0 / 62-31-7 99%View Details
1477-71-0 / 62-31-7 99%View Details
1477-71-0 / 62-31-7 -
 Dopamine hydrochloride 1477-71-0 / 62-31-7 98%View Details
Dopamine hydrochloride 1477-71-0 / 62-31-7 98%View Details
1477-71-0 / 62-31-7 -
 Dopamine hydrochloride 98%View Details
Dopamine hydrochloride 98%View Details
1477-71-0 / 62-31-7 -
 Dopamine Hydrochloride (Dopamine HCl) extrapure CAS 62-31-7View Details
Dopamine Hydrochloride (Dopamine HCl) extrapure CAS 62-31-7View Details
62-31-7 -
 Dopamine hydrochloride 99% (HPLC) CAS 62-31-7View Details
Dopamine hydrochloride 99% (HPLC) CAS 62-31-7View Details
62-31-7 -
 Dopamine Hydrochloride (Dopamine HCl) 98% CAS 62-31-7View Details
Dopamine Hydrochloride (Dopamine HCl) 98% CAS 62-31-7View Details
62-31-7 -
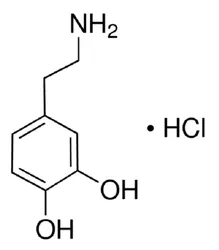 Dopamine Hydrochloride (Dopamine HCL) (CAS Number: 62-31-7), 1 kg / 5 kg / 10 kg / 25 kgView Details
Dopamine Hydrochloride (Dopamine HCL) (CAS Number: 62-31-7), 1 kg / 5 kg / 10 kg / 25 kgView Details
62-31-7
