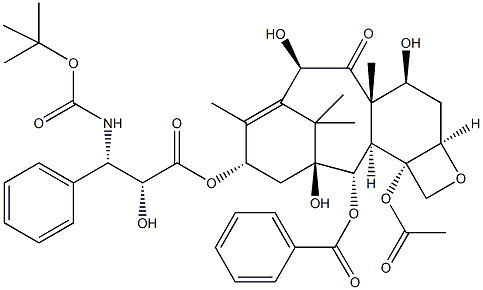
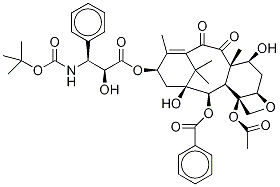
Docetaxel
- CAS NO.:125354-16-7
- Empirical Formula: C45H55NO15
- Molecular Weight: 849.93
- MDL number: MFCD09028033
- EINECS: 1533716-785-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
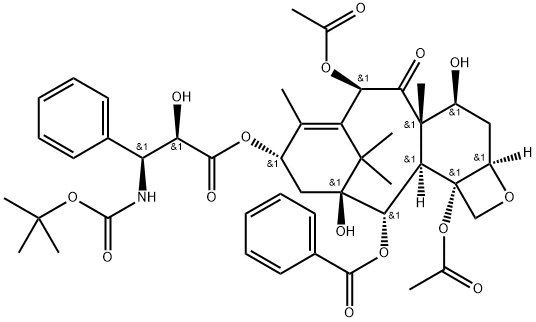
What is Docetaxel?
The Uses of Docetaxel
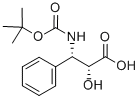
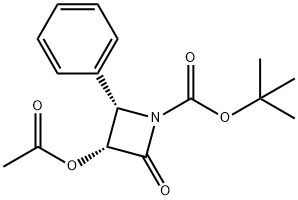
Docetaxal is an impurity of Docetaxel (D494420), a semisynthetic derivative of Paclitaxel (P132500). Docetaxel is an antimitotic agent that promotes the assembly of micro-tubules and inhibits their de -polymerization to free tubulin.
The Uses of Docetaxel
Docetaxal (Docetaxel EP Impurity G) is an impurity of Docetaxel (D494420), a semisynthetic derivative of Paclitaxel (P132500). Docetaxel is an antimitotic agent that promotes the assembly of micro-tubules and inhibits their de-polymerization to free tubulin.
What are the applications of Application
Docetaxal is an impurity of docetaxel
General Description
Docetaxel is available in single-dose vials of 20 mg/0.5 mLand 80 mg/2 mL for IV administration in the treatment of breast, NSCLC, and prostate cancers. It has also been utilizedin non–FDA-approved treatment of head, neck, gastric,bladder, and refractory ovarian cancers.
Docetaxel is highly plasma protein bound (80%) andwidely distributed with the highest concentration in the hepatobiliarysystem, but it does not appear to cross the bloodbrainbarrier. The metabolism of docetaxel has been lesswell studied than that of paclitaxel. The use of human livermicrosomes has indicated that metabolism involves oxidationof one of the tert-butyl methyl groups of the C-13 sidechain to initially give the alcohol. Further oxidation to thealdehyde and carboxylic acid both of which may cyclizewith the carbamate nitrogen to give stereoisomeric hydroxyoxazolidinonesand an oxazolidinedione, respectively. Noactive metabolites have been identified. The major enzymeinvolved is CYP3A4. The drug is primarily eliminated in thefeces with a terminal half-life of 11 hours.
The adverse effects profile for docetaxel is similar to thatof paclitaxel but also includes reversible fluid retention that isdose related. This has been associated with an initial increasein capillary permeability followed by a decrease in lymphaticdrainage later in the therapy. Restriction of sodium intake andpretreatment with corticosteroids is usually successful inminimizing this adverse effect. Peripheral neuropathy is seenwith docetaxel but occurs less often than with paclitaxel.Fatigue and muscle pain are commonly seen, and fever mayoccur in up to 30% of patients who are infection free.
Properties of Docetaxel
| Melting point: | 201-203 °C(Solv: methanol (67-56-1)) |
| Boiling point: | 901.4±65.0 °C(Predicted) |
| Density | 1.36±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 11.20±0.46(Predicted) |
| color | White to Off-White |
Safety information for Docetaxel
Computed Descriptors for Docetaxel
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
![2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
