DL-Mevalonolactone
Synonym(s):(±)-β-Hydroxy-β-methyl-δ-valerolactone;(±)-3-Hydroxy-3-methyl δ-valerolactone;DL -Mevalolactone;DL -Mevalonic acid lactone
- CAS NO.:674-26-0
- Empirical Formula: C6H10O3
- Molecular Weight: 130.14
- MDL number: MFCD00006648
- EINECS: 211-615-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
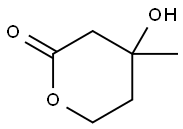
What is DL-Mevalonolactone?
Description
DL-Mevalonolactone is the δ-lactone form of mevalonic acid, a precursor in the mevalonate pathway. It induces depolarization and swelling, decreases NADPH content and aconitase (ACO) activity, and increases malondialdehyde (MDA) production in calcium-loaded rat brain mitochondria. DL-Mevalonolactone reverses decreases in glucose uptake induced by simvastatin (Item Nos. 10010344 | 10010345) in L6 myotubes. Tissue levels and urinary excretion of DL-mevalonolactone are increased in patients with mevalonic aciduria, a disorder characterized by a deficiency in mevalonic kinase activity.
Chemical properties
Pale Yellow Oil
The Uses of DL-Mevalonolactone
A metabolite from endophytes of the medicinal plant Erythrina crista-galli.
The Uses of DL-Mevalonolactone
HMG CoA substrate
The Uses of DL-Mevalonolactone
(±)-Mevalonolactone is used to:
- study the effect of statin on the prenylation of Ras and Rho GTPases
- analyse the isoprenoid biosynthesis pathways in Listeria monocytogenes
- study the the effects of statins on proliferation and migration of HUVECs (HGF-induced human umbilical vein endothelial cells)
What are the applications of Application
DL-Mevalonolactone is a useful substrate for biological research purposes
Definition
ChEBI: 4-hydroxy-4-methyl-2-oxanone is a delta-lactone.
Synthesis Reference(s)
Journal of the American Chemical Society, 104, p. 5486, 1982 DOI: 10.1021/ja00384a040
Synthesis, p. 719, 1974 DOI: 10.1055/s-1974-23416
General Description
Alkaline hydrolysis of mevalonolactone gives mevalonate. Mevalonate is a precursor of farnesyl and geranylgeranyl pyrophosphates. These pyrophosphates are required for protein prenylation.
Purification Methods
Purify the lactone via the dibenzyl-ethylenediammonium salt (m 124-125o) [Hofmann et al. J Am Chem Soc 79 2316 1957], or by chromatography on paper or on a Dowex-1 (formate) column. [Bloch et al. J Biol Chem 234 2595 1959.] Store it as the N,N'-dibenzylethylenediamine (DBED) salt, or as the lactone in a sealed container at 0o. [Beilstein 18/1 V 19.]
Properties of DL-Mevalonolactone
| Melting point: | 28 °C(lit.) |
| Boiling point: | 145-150 °C5 mm Hg(lit.) |
| alpha | -1.0~+1.0°(20℃/D)(c=5, C2H5OH) |
| Density | 1.1066 (rough estimate) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | -20°C |
| solubility | Chloroform (Sparingly), DMSO (Slightly), Ethyl Acetate, Methanol (Slightly) |
| form | Oil |
| pka | 13.57±0.20(Predicted) |
| color | Pale Yellow to Dark Brown |
| Merck | 14,6163 |
| BRN | 80960 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 674-26-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Dl-mevalonic acid lactone(674-26-0) |
Safety information for DL-Mevalonolactone
Computed Descriptors for DL-Mevalonolactone
| InChIKey | JYVXNLLUYHCIIH-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran



You may like
-
 Dl-mevalonic acid lactone 95% CAS 674-26-0View Details
Dl-mevalonic acid lactone 95% CAS 674-26-0View Details
674-26-0 -
 DL-Mevalonolactone CAS 674-26-0View Details
DL-Mevalonolactone CAS 674-26-0View Details
674-26-0 -
 (±)-Mevalonolactone CAS 674-26-0View Details
(±)-Mevalonolactone CAS 674-26-0View Details
674-26-0 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5