DL-Methionine
Synonym(s):(±)-2-Amino-4-(methylmercapto)butyric acid;DL -2-Amino-4-(methylthio)butanoic acid;DL -Methionine
- CAS NO.:59-51-8
- Empirical Formula: C5H11NO2S
- Molecular Weight: 149.21
- MDL number: MFCD00063097
- EINECS: 200-432-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:56
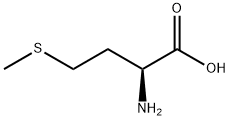
What is DL-Methionine?
Description
Methionine is an α-amino acid with the chemical formula HO2CCH(NH2)CH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the initiation codon AUG which indicates mRNA's coding region where translation into protein begins.
Chemical properties
White crystalline powder
Chemical properties
White, crystalline platelets or powder having a characteristic odor. One g dissolves in about 30 mL of water. It is soluble in dilute acids and in solutions of alkali hydroxides. It is very slightly soluble in alcohol, and practically insoluble in ether. It is optically inactive. The pH of a 1 in 100 solution is between 5.6 and 6.1. This substance may be prepared by addition of methanethiol to acrolein; by chemical conversion of methylthiopropionic aldehyde.
Chemical properties
d,l-Methionine has a characteristic odor. It is an essential amino acid and is also used as a nutrient and dietary supplement.
Occurrence
High levels of methionine can be found in eggs, sesame seeds, Brazil nuts, fish, meats and some other plant seeds; methionine is also found in cereal grains. Most fruits and vegetables contain very little of it. Most legumes are also low in methionine. Racemic methionine is sometimes added as an ingredient to pet foods.
The Uses of DL-Methionine
DL-Methionine is sometimes given as a supplement to dogs; it helps keep dogs from damaging grass by reducing the pH of the urine.
Methionine is allowed as a supplement to organic poultry feed under the US certified organic program.
The Uses of DL-Methionine
An essential nonpolar amino acid with oxidative stress defense properties
The Uses of DL-Methionine
Production of volatile compounds related to the flavour of foods from the Strecker degradation of DL-methionine. Addition of methionine in appropriate amounts to these foods might be expected to improve protein value. Economically, DL-methionine would be preferable.
Background
A preparation of methionine that includes a mixture of D-methionine and L-methionine isomers.
What are the applications of Application
DL-Methionine is an essential nonpolar amino acid with oxidative stress defense properties
Definition
ChEBI: A sulfur-containing amino acid that is butyric acid bearing an amino substituent at position 2 and a methylthio substituent at position 4.
Preparation
By addition of methanethiol to acrolein; by chemical conversion of methylthiopropionic aldehyde.
brand name
Pedameth (Forest).
Biosynthesis
As an essential amino acid, methionine is not synthesized de novo in humans, who must ingest methionine or methioninecontaining proteins. In plants and microorganisms, methionine is synthesized via a pathway that uses both aspartic acid and cysteine. First, aspartic acid is converted via β-aspartyl-semialdehyde into homoserine, introducing the pair of contiguous methylene groups. Homoserine converts to O-succinyl homoserine, which then reacts with cysteine to produce cystathionine, which is cleaved to yield homocysteine. Subsequent methylation of the thiol group by folates affords methionine. Both cystathionine-γ-synthase and cystathionine- β-lyase require pyridoxyl-5′-phosphate as a cofactor, whereas homocysteine methyltransferase requires vitamin B12 as a cofactor.
Biotechnological Production
DL-Methionine is the second amino acid that is almost exclusively manufactured by chemical synthesis. The process used today was originally developed by Werner Schwarze at Degussa in the 1940s, and has been continually improved and refined since. Today DL-methionine is manufactured in several plants, each with a capacity of more than 100,000 tonnes. To operate the process on an industrial scale also requires back-integration into the key hazardous raw materials acrolein, methyl mercaptan, and hydrogen cyanide. After the formation of the hydantoin, the key step is alkaline hydrolysis of the hydantoin, to produce methionine directly in up to 95 % yield based on acrolein.
Biological Functions
Together with cysteine, methionine is one of two sulfurcontaining proteinogenic amino acids. Its derivative S-adenosyl methionine (SAM) serves as a methyl donor. Methionine is an intermediate in the biosynthesis of cysteine, carnitine, taurine, lecithin, phosphatidylcholine, and other phospholipids. Improper conversion of methionine can lead to atherosclerosis.
This amino acid is also used by plants for synthesis of ethylene. The process is known as the Yang Cycle or the methionine cycle.
Methionine is one of only two amino acids encoded by a single codon (AUG) in the standard genetic code (tryptophan, encoded by UGG, is the other). The codon AUG is also the most common eukaryote "Start" message for a ribosome that signals the initiation of protein translation from mRNA when the AUG codon is in a Kozak consensus sequence. As a consequence, methionine is often incorporated into the N-terminal position of proteins in eukaryotes and archaea during translation, although it can be removed by post-translational modification. In bacteria, the derivative Nformylmethionine is used as the initial amino acid.
General Description
DL-Methionine is an essential amino acid containing sulphur. Methionine consists of an asymmetric carbon and exists as D (dextrogyre) and L (levogyre) optical isomers. The L-methionine is considered as biologically active. The racemic mixture of D and L-isomers forms DL-methionine, which is the commercially available methionine.
Biochem/physiol Actions
Methionine offers protection against disorders related to hair, skin and nails. It elevates lecithin production in the liver and thereby reduces cholesterol level. It naturally serves as a heavy metal chelating agent, regulating ammonia concentration in the urine. This ameliorates bladder irritation. Methionine is also known to induce hair growth.
Safety Profile
Moderately toxic by ingestion and other routes. An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of SOx and NOx. See also 1-METHIONINE.
Benefits
DL-Methionine is an essential amino acid involved in synthesizing proteins and other vital compounds in the body. By supporting protein synthesis, DL-Methionine helps to build and repair tissues, including muscles, skin, hair, and nails. It is beneficial for liver health by reducing the buildup of excess fat. This can help to prevent liver damage and support overall liver function. DL-Methionine plays a role in synthesizing the antioxidant glutathione, which helps protect the body from oxidative damage caused by free radicals. This can help to prevent cell damage and support overall health and well-being. In addition, it has been shown to positively impact wound healing. It may help to reduce oxidative damage, support the formation of new tissue, and promote the healing process.
Side Effects
Side effects may include Loss of appetite, Vomiting, and Loss of balance.
Purification Methods
Crystallise it from hot water or EtOH. Also purify it by dissolving it in H2O and passing through an Amberlite IR-120 resin (NH4+ form). The eluate is concentrated and then passed through Amberlite IR-4B resin, and this eluate is evaporated to dryness. The residue is washed with EtOH, then Me2CO, dried and recrystallised from aqueous EtOH (colourless plates) [Baddiley & Jamieson J Chem Soc 4283 1954]. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 2125 1961, Beilstein 4 IV 3190.]
Other biochemical pathways
Although mammals cannot synthesize methionine, they can still use it in a variety of biochemical pathways:
Generation of homocysteine Methionine is converted to S-adenosyl methionine (SAM) by (1) methionine adenosyltransferase.
SAM serves as a methyl-donor in many (2) methyl transferase reactions, and is converted to S-adenosylhomocysteine (SAH).
(3) Adenosyl homocysteinase converts SAH to homocysteine.
There are two fates of homocysteine: it can be used to regenerate methionine, or to form cysteine.
Regeneration of methionine
Methionine can be regenerated from homocysteine via methionine synthase in a reaction that requires Vitamin B12 as a cofactor.
Homocysteine can also be remethylated using glycine betaine (NNN-trimethyl glycine, TMG) to methionine via the enzyme betainehomocysteine methyltransferase (E.C.2.1.1.5, BHMT). BHMT makes up to 1.5% of all the soluble protein of the liver, and recent evidence suggests that it may have a greater influence on methionine and homocysteine homeostasis than methionine synthase.
Properties of DL-Methionine
| Melting point: | 284 °C (dec.)(lit.) |
| Boiling point: | 306.9±37.0 °C(Predicted) |
| alpha | -1~+1°(D/20℃)(c=8,HCl) |
| Density | 1.34 |
| refractive index | 1.5216 (estimate) |
| FEMA | 3301 | D,L-METHIONINE |
| storage temp. | 2-8°C |
| solubility | 1 M HCl: 0.5 M at 20 °C, clear, colorless |
| pka | 2.13(at 25℃) |
| form | Crystals or Crystalline Powder |
| color | White |
| Odor | mild sulfurous acidic |
| optical activity | [α]/D, c = 5 in 5 M HCl (inactive) |
| Water Solubility | 2.9 g/100 mL (20 ºC) |
| Merck | 14,5975 |
| JECFA Number | 1424 |
| BRN | 636185 |
| Stability: | Stable. Incompatible with strong oxidising agents. |
| CAS DataBase Reference | 59-51-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Methionine(59-51-8) |
| EPA Substance Registry System | Methionine (59-51-8) |
Safety information for DL-Methionine
Computed Descriptors for DL-Methionine
| InChIKey | FFEARJCKVFRZRR-UHFFFAOYSA-N |
DL-Methionine manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Dl-Methionine Feed Grade 99%View Details
Dl-Methionine Feed Grade 99%View Details -
 DL-Methionine extrapure CHR CAS 59-51-8View Details
DL-Methionine extrapure CHR CAS 59-51-8View Details
59-51-8 -
 DL-Methionine CAS 59-51-8View Details
DL-Methionine CAS 59-51-8View Details
59-51-8 -
 D L Methionine(Feed Grade) CASView Details
D L Methionine(Feed Grade) CASView Details -
 DL-Methionine 98% CAS 59-51-8View Details
DL-Methionine 98% CAS 59-51-8View Details
59-51-8 -
 D L Methionine Pharma Grade CASView Details
D L Methionine Pharma Grade CASView Details -
 DL-MethionineView Details
DL-MethionineView Details
59-51-8 -
 Methionine Food Chemical, 149.21 G Mol-1, C5H11NO2SView Details
Methionine Food Chemical, 149.21 G Mol-1, C5H11NO2SView Details
59-51-8
