DISOPYRAMIDE
Synonym(s):α-Diisopropylaminoethyl-α-phenylpyridine-2-acetamide
- CAS NO.:3737-09-5
- Empirical Formula: C21H29N3O
- Molecular Weight: 339.48
- MDL number: MFCD00057366
- EINECS: 223-110-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
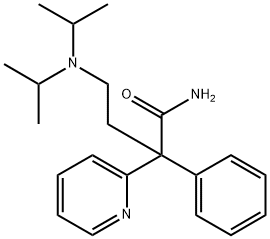
What is DISOPYRAMIDE?
Absorption
Nearly complete
Toxicity
LD50=580 mg/kg in rats
Description
Structurally, disopyramide does not belong to any of the known classes of antiarrhythmics; however, being a drug of the class IA sodium channel blockers, it exhibits membranestabilizing action and increases the effective refractory period and duration of an action potential in the atrium and ventricles. It causes a decrease in contractability and excitability of the myocardium, slowing of conductivity, and suppression of sinoatride automatism.
Chemical properties
Crystalline Solid
Originator
Rythmodan,Cassenne,France,1969
The Uses of DISOPYRAMIDE
Disopyramide is used for preventing and restoring atrial and ventricular extrasystole and tachycardia in order to prevent atrial flutter and arrhythmia.
The Uses of DISOPYRAMIDE
Antiarrhythmic (class IA). Sodium channel blocker
The Uses of DISOPYRAMIDE
Antiarrhythmic;Na+ channel blocker
Indications
For the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia, ventricular pre-excitation and cardiac dysrhythmias. It is a Class Ia antiarrhythmic drug.
Background
A class I anti-arrhythmic agent (one that interferes directly with the depolarization of the cardiac membrane and thus serves as a membrane-stabilizing agent) with a depressant action on the heart similar to that of guanidine. It also possesses some anticholinergic and local anesthetic properties.
What are the applications of Application
Disopyramide is an antiarrhythmic (class IA) and a sodium channel blocker
Definition
ChEBI: A monocarboxylic acid amide that is butanamide substituted by a diisopropylamino group at position 4, a phenyl group at position 2 and a pyridin-2-yl group at position 2. It is used as a anti-arrhythmia drug.
Manufacturing Process
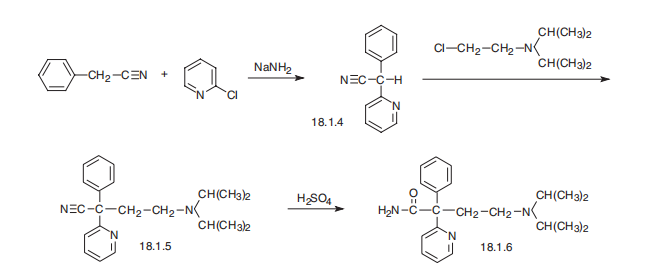
To a solution of 35.3 parts of phenylacetonitrile and 47.6 parts of 2-
bromopyridine in 175 parts of dry toluene is added 53.4 parts of sodamide
slowly with stirring over a period of 45 minutes. The resultant mixture is
stirred at 100°C for 2 hours before it is cooled and the excess sodamide is
decomposed by the addition of water. The toluene layer is separated and
washed with water to remove excess alkali. The toluene solution is extracted
with 6 N hydrochloric acid and the acid extract is made alkaline and then
extracted with toluene. The toluene solution is dried over sodium sulfate and
the solvent is evaporated. Recrystallization of the residue from alcohol-hexane
gives α-phenyl-2-pyridineacetonitrile melting at about 87-88°C.
To a solution of 41 parts of α-phenyl-2-pyridineacetonitrile in 350 parts of dry
toluene is added 9.2 parts of sodamide and the mixture is stirred and heated
at 90°C for 30 minutes. Heating is stopped and a solution of 38.5 parts of 2-
diisopropylaminoethyl chloride in 110 parts of dry toluene is added slowly over
a period of 30 minutes. The mixture is stirred and refluxed for 6 hours before
it is cooled and decomposed by the addition of water. The toluene layer is
separated and washed with water and extracted with 6 N hydrochloric acid.
The acid extract is made alkaline and extracted with toluene. The toluene
solution is washed with water and dried and the solvent is evaporated.
Distillation of the residue gives 4-diisopropylamino-2-phenyl-2-(2-pyridyl)-
butyronitrile boiling at about 145°-160°C at 0.3 mm pressure.
A solution of 27.2 parts of 4-diisopropylamino-2-phenyl-2-(2-
pyridyl)butyronitrile in 200 parts of concentrated sulfuric acid is heated on a
steam bath for 4 hours and then poured onto ice. The resultant mixture is
alkalized with 10 N sodium hydroxide, and the pH is adjusted to 6 by the
addition of acetic acid. The solution is washed once with benzene before it is
alkalized again with 10 N sodium hydroxide solution. The resultant mixture is
extracted with benzene, and the solvent is evaporated from the benzene
extract. The resultant residue is dissolved in ethanol and the alcohol solution
is treated with charcoal and filtered. Evaporation of the solvent leaves a
residue which is recrystallized from hexane to give 4-diisopropylamino-2-
phenyl-2-(2-pyridyl)butyramide melting at about 94.5-95°C. It may be
converted to the phosphate with phosphoric acid.
brand name
Norpace (Searle).
Therapeutic Function
Antiarrhythmic
Mechanism of action
Disopyramide has a pharmacological profile similar to that of quinidine and procainamide . Clinically, it is active against most forms of arrhythmias (supraventricular and ventricular).
Pharmacokinetics
Disopyramide is an anti-arrhythmic drug indicated for the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia that are life-threatening. At therapeutic plasma levels, disopyramide shortens the sinus node recovery time, lengthens the effective refractory period of the atrium, and has a minimal effect on the effective refractory period of the AV node. Little effect has been shown on AV-nodal and His-Purkinje conduction times or QRS duration. However, prolongation of conduction in accessory pathways occurs.
Pharmacokinetics
Disopyramide phosphate is used orally for the treatment of certain ventricular and atrial arrhythmias. Despite its structural dissimilarity to procainamide, its cardiac effects are very similar. Disopyramide is rapidly and completely absorbed from the gastrointestinal tract. Peak plasma level is usually reached within 1 to 3 hours, and a plasma half-life of 5 to 7 hours is common. Approximately half of an oral dose is excreted unchanged in the urine. The remaining drug undergoes hepatic metabolism, principally to the corresponding N-dealkylated form. This metabolite retains approximately half the antiarrhythmic activity of disopyramide and also is subject to renal excretion.
Clinical Use
Disopyramide (Norpace) can suppress atrial and ventricular
arrhythmias and is longer acting than other
drugs in its class.
The indications for use of disopyramide are similar to
those for quinidine, except that it is not approved for
use in the prophylaxis of atrial flutter or atrial fibrillation
after DC conversion.The indications are as follows:
unifocal premature (ectopic) ventricular contractions,
premature (ectopic) ventricular contractions of multifocal
origin, paired premature ventricular contractions
(couplets), and episodes of ventricular tachycardia.
Persistent ventricular tachycardia is usually treated with
DC conversion.
Side Effects
The major toxic reactions to disopyramide administration
include hypotension, congestive heart failure, and
conduction disturbances. These effects are the result of
disopyramide’s ability to depress myocardial contractility
and myocardial conduction. Although disopyramide
initially may produce ventricular tachyarrhythmias or
ventricular fibrillation in some patients, the incidence of
disopyramide-induced syncope in long-term therapy is
not known. Most other toxic reactions (e.g., dry mouth,
blurred vision, constipation) can be attributed to the anticholinergic
properties of the drug.
CNS stimulation and hallucinations are rare.The incidence
of severe adverse effects in long-term therapy
may be lower than those observed with quinidine or
procainamide.
Synthesis
Disopyramide, |á-(2-diisopropylaminoethyl)-|á-phenyl-2-pyridineacetamide (18.1.6), is synthesized by arylating benzylcyanide with 2-chloropiridine in the presence of sodium amide and subsequent alkylation of the resulting |á-phenyl-|á-(2-pyridyl) acetonitrile (18.1.4) with 2-diisopropylaminoethylchloride using sodium amide. Sulfuric acid hydrolysis of the resulting nitrile (18.1.5) leads to the formation of |á-(2-diisopropylaminoethyl)- |á-phenyl-2-pyridineacetamide, disopyramide.
Drug interactions
In the presence of phenytoin, the metabolism of disopyramide
is increased (reducing its effective concentration)
and the accumulation of its metabolites is also
increased, thereby increasing the probability of anticholinergic
adverse effects. Rifampin also stimulates the
hepatic metabolism of disopyramide, reducing its
plasma concentration.
Unlike quinidine, disopyramide does not increase
the plasma concentration of digoxin in patients receiving
a maintenance dose of the cardiac glycoside.
Hypoglycemia has been reported with the use of
disopyramide, particularly in conjunction with moderate
or excessive alcohol intake.
Metabolism
Hepatic
Precautions
Disopyramide should not be administered in cardiogenic
shock, preexisting second- or third-degree A-V
block, or known hypersensitivity to the drug. Neither
should it be given to patients who are poorly compensated
or those with uncompensated heart failure or severe
hypotension. Because of its ability to slow cardiac
conduction, disopyramide is not indicated for the treatment
of digitalis-induced ventricular arrhythmias.
Patients with congenital prolongation of the QT interval
should not receive quinidine, procainamide, or disopyramide
because further prolongation of the QT interval
may increase the incidence of ventricular fibrillation.
Because of its anticholinergic properties, disopyramide
should not be used in patients with glaucoma.
Urinary retention and benign prostatic hypertrophy are
also relative contraindications to disopyramide therapy.
Patients with myasthenia gravis may have a myasthenic
crisis after disopyramide administration as a result of
the drug’s local anesthetic action at the neuromuscular
junction.The elderly patient may exhibit increased sensitivity
to the anticholinergic actions of disopyramide.
Caution is advised when disopyramide is used in
conjunction with other cardiac depressant drugs, such as verapamil, which may adversely affect atrioventricular
conduction.
References
1) Hell?et al.?(1978),?Disopyramide: a review of its pharmacological properties and therapeutic use in treating cardiac arrhythmias; Drugs?115?331 2) Verlinden?et al.?(2015),?Disopyramide for Hypertrophic Cardiomyopathy: A Pragmatic Reappraisal of an Old Drug; Pharmacotherapy,?35?1164 3) Nakajima?et al.?(1989),?Anti-Cholinergic Effects of Quinidine, Disopyramide, and Procainamide in Isolated Atrial Myocytes: Mediation by Different Molecular Mechanisms; Circ. Res.,?64?297
Properties of DISOPYRAMIDE
| Melting point: | 94.5-950C |
| Boiling point: | 475.43°C (rough estimate) |
| Density | 1.0779 (rough estimate) |
| refractive index | 1.6300 (estimate) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Soluble in DMSO (25 mg/ml) and Ethanol (>35 mg/mL) |
| form | solid |
| pka | 10.2; also reported as 10.45(at 25℃) |
| color | White |
| Water Solubility | 6.17mg/L(22.5 ºC) |
| Merck | 14,3360 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 3737-09-5(CAS DataBase Reference) |
Safety information for DISOPYRAMIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for DISOPYRAMIDE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran

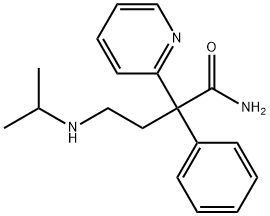
You may like
-
 0/5/37 DISOPYRAMIDE 99%View Details
0/5/37 DISOPYRAMIDE 99%View Details
0/5/37 -
 Disopyramide 95% CAS 3737-09-5View Details
Disopyramide 95% CAS 3737-09-5View Details
3737-09-5 -
 Disopyramide CAS 3737-09-5View Details
Disopyramide CAS 3737-09-5View Details
3737-09-5 -
 4-(DIISOPROPYLAMINO)-2-PHENYL-2-(2-PYRIDINYL)BUTANAMIDE CAS 3737-09-5View Details
4-(DIISOPROPYLAMINO)-2-PHENYL-2-(2-PYRIDINYL)BUTANAMIDE CAS 3737-09-5View Details
3737-09-5 -
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1