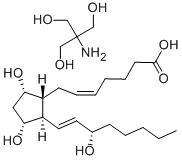
Dinoprost tromethamine
Synonym(s):(5Z,9α,11α,13E,15S)-9,11,15-Trihydroxyprosta-5,13-dienoic acid tris salt;PGF2α−Tris;Prostaglandin F2α tris salt
- CAS NO.:38562-01-5
- Empirical Formula: C24H45NO8
- Molecular Weight: 475.62
- MDL number: MFCD00077863
- EINECS: 254-002-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Dinoprost tromethamine?
Absorption
Slowly absorbed from the amniotic fluid into systemic circulation.
Toxicity
Although overdose by intra-amniotic administration of dinoprost has not been reported, exaggeration of the nausea, vomiting, and diarrhea that occur with normal doses would be expected.
Description
Dinoprost tromethamine is a synthetic analogue of prostaglandin. F2α is an autocrine hormone present in many mammalian tissues. It is used to cause luteolysis in the cow, sow and mare. It is given as a single injection by im or sc routes, the recommended dosage rate is 25 mg cow; 10 mg sow and 5 mg mare. It is rapidly absorbed from the injection site. It has an extremely short half life of only a few minutes, it is almost completely cleared following one or two passages through the liver and/or lungs.
Chemical properties
White Solid
Originator
Prostin F2A,Upjohn,UK,1972
The Uses of Dinoprost tromethamine
One of the most biologically studied of the primary prostaglandins. Closely related to Prostaglandin E2 (PGE2) in that both prostaglandins are biosynthesized from the same precursors and that PGF2 is the synthetic reduction product of PGE2. Phospholipase A2 (PLA2) is a key enzyme for biosynthesis of PGF2α. Oxytocic; abortifacient.
The Uses of Dinoprost tromethamine
One of the most biologically studied of the primary prostaglandins. Closely related to Prostaglandin E2 (PGE2) in that both prostaglandins are biosynthesized from the same precursors and that PGF2 is the synthetic reduction product of PGE2. Phospholipase A2 (PLA2) is a key enzyme for biosynthesis of PGF2α. Oxytocic; abortifacient.
Background
The tromethamine (THAM) salt of the naturally occurring prostaglandin F2 alpha, dinoprost tromethamine occurs as a white to off-white, very hygroscopic, crystalline powder. Dinoprost tromethamine may also be known as dinoprost trometamol, PGF2 alpha THAM, or prostaglandin F2 alpha tromethamine.
Indications
Used for aborting second-trimester pregnancy (between the twelfth to eighteenth week of gestation) and in incomplete abortion or for therapeutic abortion in cases of intrauterine fetal death and congenital abnormalities incompatible with life. Also used at low-doses for medically indicated induction of labor at term. Also injected intra-arterially for use as a vasodilator to assist in angiography.
What are the applications of Application
PGF2α (Prostaglandin F2α) is binds to PGF2Rα, shown to stimulate COX2 expression, and regulate adrenal endocrine function
Definition
dinoprost tromethamine is a synthetic analogue of the naturally occurring prostaglandin F2 alpha. Prostaglandin F2 alpha stimulates myometrial activity, relaxes the cervix, inhibits corpus luteal steroidogenesis, and induces luteolysis by direct action on the corpus luteum.
Indications
Dinoprost tromethamine salt (Prostaglandin F2α tromethamine salt) is an orally active, potent prostaglandin F (PGF) receptor (FP receptor) agonist. Dinoprost tromethamine salt is a luteolytic hormone produced locally in the endometrial luminal epithelium and corpus luteum (CL). Dinoprost tromethamine salt plays a key role in the onset and progression of labour.
Manufacturing Process
A solution of tris(hydroxymethyl)aminomethane (1.645 grams) in 3.0 ml of
water at 60°C is added with vigorous stirring to a solution of PGF2α, (5.00
grams) in 700 ml of acetonitrile which has just been
ought to its boiling
point. The vessel which contained the aqueous amine solution is rinsed with
three 0.66 ml portions of water, each rinsing being added with vigorous
stirring to the acetonitrile solution. The mixture is then cooled to 25°C by
immersion of the vessel in cool water. At the cloud point, the vessel wall
(glass) below the liquid surface is scratched vigorously with a glass rod. The
mixture is then maintained at 25°C for 24 hours.
The resulting crystals are collected by filtration under nitrogen, washed on the
filter with 50 ml of acetonitrile, and then dried by passing nitrogen at 50°C
through the filter cake for one hour. Drying is completed in an oven at 70°C
for 8 hours to give 5.965 grams of the tris(hydroxymethyl)aminomethane salt of PGF2α in free flowing crystalline form; MP 100°-101°C.
brand name
Prostin F2 Alpha (Pharmacia & Upjohn).
Therapeutic Function
Smooth muscle stimulant
Biochem/physiol Actions
Prostaglandin F2α is induced by uterine-produced oxytocin and acts on the corpus luteum to cause luteolysis and inhibit progesterone production.
Pharmacokinetics
Dinoprost tromethamine is the tromethamine (THAM) salt of the naturally occurring prostaglandin F2alpha. Prostaglandin F2alpha?has several pharmacologic effects on the female reproductive system, including stimulation of myometrial activity, relaxation of the cervix, inhibition of steroidogenesis by corpora lutea, and can potentially lyse corpora lutea.
Safety Profile
Poison by intraperitoneal, subcutaneous, intravenous, and intramuscular routes. Moderately toxic by ingestion. Human reproductive effects by intervagmal route: terminates pregnancy, effects on fertility. Experimental teratogenic and reproductive effects. When heated to decomposition it emits toxic fumes of NOx. See also other prostaglandin entries.
Metabolism
Enzymatic dehydrogenation primarily in the maternal lungs and also in the liver.
storage
Store at -20°C
Properties of Dinoprost tromethamine
| Melting point: | 100-101° |
| storage temp. | -20°C |
| solubility | H2O: 1 mg/mL Aqueous solutions are stable for 30 days at 2-8°C and for several months frozen at −0°C in single use aliquots. Avoid repeated freeze/thaw cycles. |
| form | powder |
| color | white |
| Merck | 13,7970 |
| BRN | 4087514 |
| Stability: | Hygroscopic |
Safety information for Dinoprost tromethamine
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Dinoprost tromethamine
| InChIKey | IYGXEHDCSOYNKY-RZHHZEQLSA-N |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Dinoprost tromethamine CAS 38562-01-5View Details
Dinoprost tromethamine CAS 38562-01-5View Details
38562-01-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1