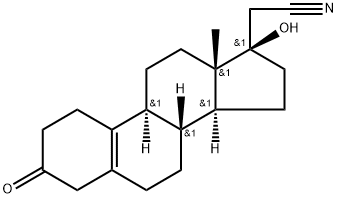
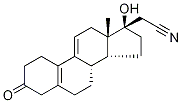
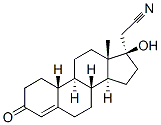
Dienogestrel
Synonym(s):(17a)-17-Hydroxy-3-oxo-19-norpregna-4,9-diene-21-nitrile;17-cyanomethyl-17-hydroxy-estra-4,9-dien-3-one;17-Hydroxy-3-oxo-19-norpregna-4,9-diene-21-nitrile
- CAS NO.:65928-58-7
- Empirical Formula: C20H25NO2
- Molecular Weight: 311.43
- MDL number: MFCD00868356
- EINECS: 639-448-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22

What is Dienogestrel ?
Absorption
Dienogest is rapidly absorbed following oral administration, with 91% bioavailability. The peak plasma concentration of 47 ng/mL is reached at about 1.5 hours after single ingestion of 2 mg . The stable concentrations of the drug are reached after two days of initial treatment .
Toxicity
Oral LD50 in mouse is 4 mg/kg . In a long-term carcinogenicity study involving rats and mice, exposure of 10 times the dose of maximum recommended clinical dose of dienogest resulted in increased incidences of pituitary adenomas, fibroepithelial mammary tumours, stromal polyps of the uterus and malignant lymphoma . These tumors are thought to arise from marked species differences in the optimal oestrogen:progestogen ratio for reproductive function. In rat liver foci assay, dienogest did not induce tumor promotion activity . Dienogest does not display genotoxic potential.
Physical properties
Dienogestrel is a pale yellow solid.
The Uses of Dienogestrel
Dienogest is an orally active synthetic progesterone (or progestin). It is available for use as an oral contraceptive in combination with ethinylestradiol. It has antiandrogenic activity and as a result can improve androgenic symptoms.
The Uses of Dienogestrel
Derivative of 19-Nortestosterone. In combination with estrogen as oral contraceptive. Progestogen.
Indications
Indicated for use as the treatment of endometriosis alone and as a contraceptive in combination with ethinylestradiol.
Background
Dienogest is an orally-active semisynthetic progestogen which also possesses the properties of 17α-hydroxyprogesterone. It is a derivative of 19-nortestosterone and has antiandrogenic properties. It is primarily used as a contraceptive in combination with ethinylestradiol, or in other combination form pills approved in United States and Europe however it is not available in the US by itself. In Europe, Australia, Malaysia, Singapore and Japan, dienogest single therapy is an approved treatment for endometriosis to alleviate painful symptoms of endometriosis and reduce endometriotic lesions . Dienogest is commonly marketed as Visanne, Natazia and Qlaira.
What are the applications of Application
Dienogest is a progesterone 19-nortestosterone derivative
Definition
ChEBI: A steroid hormone that is 17beta-hydroxy-3-oxoestra-4,9-diene substituted at position 17 by a cyanomethyl group. Used as an oral contraceptive.
brand name
Endometrion (Schering A.G., Germany).
Biochem/physiol Actions
Dienogest is used to treat endometriosis. It might also help to decrease the levels of plasma estradiol by stimulating cell death of granulosa cells in the ovary.
Pharmacokinetics
Dienogest exhibits a very potent progestagenic effect in the endometrium, and causes endometrial atrophy after prolonged use . It also mediates an antiandrogenic effect that is equivalent to approximately one third that of cyproterone acetate . A dose of 2 mg inhibits the growth of ovarian follicles at 10 mm and maintains the concentration of progesterone at a low level, but has a weak inhibitory effect on FSH and LH. 1mg/kg of dienogest also directly inhibits ovulation . In clinical trials composing of patients with endometriosis, dienogest therapy effectively reduced painful symptoms and endometriotic lesions associated with the disorder . Dienogest displays no antiestrogenic activity as it activate neither estrogen receptor (ER) α nor ERβ , and causes hypoestrogenic effects instead as it is shown to decrease the relative expressions of ERβ and ERα . It has no glucocorticoid or mineralocorticoid effects. In combined oral contraceptive pills (COCP) with ethinyloestradiol, dienogest conjuction therapy effectively reduces the symptoms of acne and hirsutism, as well as improving excessively heavy or prolonged menstrual bleeding .
Metabolism
Dienogest undergoes complete metabolism that is mainly mediated by CYP3A4. The metabolites are pharmacologically inactive and rapidly eliminated from the plasma.
Properties of Dienogestrel
| Melting point: | 210-2140C |
| Boiling point: | 451.46°C (rough estimate) |
| alpha | D25 -290° (c = 0.5 in pyridine) |
| Density | 1.0899 (rough estimate) |
| refractive index | 1.5614 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Pyridine (Slightly) |
| form | powder |
| pka | 13?+-.0.40(Predicted) |
| color | white to beige |
| optical activity | [α]/D -305 to -325°, c = 1 in dichloromethane |
| Merck | 14,3106 |
| InChI | InChI=1/C20H25NO2/c1-19-8-6-16-15-5-3-14(22)12-13(15)2-4-17(16)18(19)7-9-20(19,23)10-11-21/h12,17-18,23H,2-10H2,1H3/t17-,18+,19+,20-/s3 |
| CAS DataBase Reference | 65928-58-7(CAS DataBase Reference) |
Safety information for Dienogestrel
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Dienogestrel
| InChIKey | AZFLJNIPTRTECV-XAYGXGNENA-N |
| SMILES | C[C@]12CCC3=C4CCC(=O)C=C4CC[C@@]3([H])[C@]1([H])CC[C@@]2(O)CC#N |&1:1,14,16,20,r| |
Dienogestrel manufacturer
Swati Spentose Pvt Ltd
BDR Pharmaceuticals International Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![(17α)-3,3-[1,2-Ethanediylbis(oxy)]-17-hydroxy-19-norpregna-5(10),9(11)-diene-21-nitrile](https://img.chemicalbook.in/CAS/GIF/190662-30-7.gif)
You may like
-
 Dienogest 99%View Details
Dienogest 99%View Details -
 65928-58-7 99%View Details
65928-58-7 99%View Details
65928-58-7 -
 Dienogestrel 98% (HPLC) CAS 65928-58-7View Details
Dienogestrel 98% (HPLC) CAS 65928-58-7View Details
65928-58-7 -
 Dienogest CAS 65928-58-7View Details
Dienogest CAS 65928-58-7View Details
65928-58-7 -
 Dienogest CAS 65928-58-7View Details
Dienogest CAS 65928-58-7View Details
65928-58-7 -
 65928-58-7 98%View Details
65928-58-7 98%View Details
65928-58-7 -
 Dienogest CAS 65928-58-7View Details
Dienogest CAS 65928-58-7View Details
65928-58-7 -
 Dienogest for system suitability CAS 65928-58-7View Details
Dienogest for system suitability CAS 65928-58-7View Details
65928-58-7