Dimethylglyoxime
Synonym(s):DMG;Dimethylglyoxime;2,3-Butanedione dioxime;Diacetyldioxime;Diacetyldioxime, 2,3-Butanedionedioxime disodium salt
- CAS NO.:95-45-4
- Empirical Formula: C4H8N2O2
- Molecular Weight: 116.12
- MDL number: MFCD00002117
- EINECS: 202-420-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-03 21:42:24
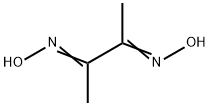
What is Dimethylglyoxime?
Chemical properties
Dimethylglyoxime is a white crystalline powder. Soluble in alcohol, ether, acetone and pyridine. Solubility in water 0.5g/l, insoluble in tolerate chloroform, toluene, xylene. It is one of the first selective organic reagents applied in analytical chemistry. It is an extraordinary sensitive and specific reagent for nickel. under appropriate conditions dimethylglyoxime is specific for nickel(II) and palladium(II), but it also forms coloured water-soluble complexes with iron(II), cobalt(II) and copper(II).
The Uses of Dimethylglyoxime
Dimethylglyoxime is specific for nickel(II) ions in ammoniacal solution if alkali tartrate is employed as masking agent to keep in solution the possibly interfering hydrolysing salts.
What are the applications of Application
Dimethylglyoxime is a chelating agent which forms complexes with metals such as copper, nickel, and palladium.
Reactions
Nickel cation reacts with dimethylglyoxime forms an insoluble red precipitate of nickel dimethylglyoxime.
Ni2+ + 2C4H8N2O2 → Ni(C4H7N2O2)2↓(red precipitate) + 2H+
Dimethylglyoxime reacts with ferrous sulphate and ammonium hydroxide forms a complex compound of iron and ammonium sulphate and water is formed.
FeSO4 + 2NH4OH + 2C4H8N2O2 → Fe(C4H7N2O2)2 + (NH4)2SO4 + 2H2O
General Description
Dimethylglyoxime (DMG) is a complexing ligand. Dimethylglyoxime forms a number of mixed ligand complexes with N-acetylglycine with metals such as VO(IV), Ni(II), Zn(II), Pd(II), Cd(II) and Pb(II). It is a useful reagent for the spectrophotometric determination of Co(II), Fe(II), Ni(II), Pd(II) and Re(VII).
Applications
Dimethylglyoxime is defined as a well-known ligand used in the analysis of nickel, forming an insoluble red complex with nickel known as Ni(DMGH)2. It provides a planar tetracoordinate nitrogen environment analogous to that found in the corrin ring of vitamin B12 and the porphyrin ring of heme systems.
Detection use
The dimethylglyoxime test (nickel spot test) and the cobalt spot test (based on disodium-1-nitroso-2-naphthol-3,6-disulfonate) can be used to detect nickel or cobalt released from metal objects and dermal exposure, thus aiding in avoidance of contact in sensitized patients.
Properties of Dimethylglyoxime
| Melting point: | 240-241 °C(lit.) |
| Boiling point: | 217.15°C (rough estimate) |
| Density | 1.2829 (rough estimate) |
| refractive index | 1.4880 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in alcohol, acetone, ether |
| form | Powder |
| pka | pK1:10.60 (25°C) |
| color | White to off-white |
| Odor | Odorless |
| Water Solubility | INSOLUBLE |
| Merck | 14,3246 |
| BRN | 506731 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 95-45-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 2,3-Butanedione, dioxime(95-45-4) |
| EPA Substance Registry System | 2,3-Butanedione, dioxime (95-45-4) |
Safety information for Dimethylglyoxime
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for Dimethylglyoxime
| InChIKey | JGUQDUKBUKFFRO-GGWOSOGESA-N |
Dimethylglyoxime manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran






You may like
-
 DIMETHYL GLYOXIME 99%View Details
DIMETHYL GLYOXIME 99%View Details -
 Dimethylglyoxime CAS 95-45-4View Details
Dimethylglyoxime CAS 95-45-4View Details
95-45-4 -
 Dimethylglyoxime CAS 95-45-4View Details
Dimethylglyoxime CAS 95-45-4View Details
95-45-4 -
 Dimethylglyoxime extrapure AR CAS 95-45-4View Details
Dimethylglyoxime extrapure AR CAS 95-45-4View Details
95-45-4 -
 Dimethylglyoxime 99% (HPLC) CAS 95-45-4View Details
Dimethylglyoxime 99% (HPLC) CAS 95-45-4View Details
95-45-4 -
 Dimethylglyoxime, Analytical Grade, Bottle of 500 gmView Details
Dimethylglyoxime, Analytical Grade, Bottle of 500 gmView Details
95-45-4 -
 Dimethylglyoxime LR, Laboratory Reagent Grade (LR)View Details
Dimethylglyoxime LR, Laboratory Reagent Grade (LR)View Details
95-45-4 -
 Dimethyl Glyoxime PowderView Details
Dimethyl Glyoxime PowderView Details
95-45-4
