Diisopropyl azodicarboxylate
Synonym(s):DIAD;Diisopropyl azodiformate;Diisopropylazodicarboxylate
- CAS NO.:2446-83-5
- Empirical Formula: C8H14N2O4
- Molecular Weight: 202.21
- MDL number: MFCD00008875
- EINECS: 219-502-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-20 17:40:16
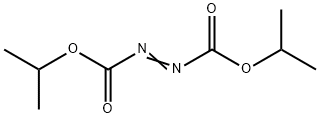
What is Diisopropyl azodicarboxylate?
Description
Diisopropyl azodicarboxylate (DIAD) is typically used for Mitsunobu reactions. Similar reagents to DIAD include DEAD, DtBAD, and ADDP. DIAD is quickly replacing Diethyl azodicarboxylate (DEAD) as the preferred azodicarboxylate due to the known safety concerns of DEAD.
Chemical properties
CLEAR ORANGE LIQUID
The Uses of Diisopropyl azodicarboxylate
Diisopropyl Azodicarboxylate is an azodicarboxylic acid derivative used as a reagent in producing many organic compounds. Diisopropyl Azodicarboxylate is commonly used as an oxidizer in Mitsun obu reactions. It can also serve as a selective deprotectant of N-benzyl groups in the presence of other protecting groups. It acts as a reactant in preparing chromenes resembling classical cannabinoids, norbornene-based guanidine-rich polymers and acceptor-donor-acceptor organic dyes.
General Description
Reacted with dioxaphosphorinanes and iron catalyst to generate rearranged phosphonium ylide products.
Synthesis
A synthesis method of diisopropyl azodicarboxylate comprises the following steps: (1) adding alkali and a solvent under the protection of inert gases, sequentially adding carbazic acid isopropyl ester and diethyl carbonate, carrying out the heating reaction at 100-180 ℃ for 2-6 hours, adjusting the pH value of a solution to 4.5-7.5 with acid, and carrying out suction filtration, to obtain hydrogenated diisopropyl azodiformate; and (2) adding 40%-50% of sulfuric acid solution to the hydrogenated diisopropyl azodiformate at -20 ℃ to 25 ℃, dissolving, adding a catalyst, controlling the reaction temperature to -15 ℃ to -5 ℃, dropwise adding 10%-15% of hydrogen peroxide until no heat is emitted, further reacting for 5-10 hours after dropwise adding is ended, quenching, extracting, washing to be neutral, drying, filtering, and concentrating on obtaining the diisopropyl azodicarboxylate.
Purification Methods
Purify the azo compound by distillation at as high a vacuum as possible. Since it is likely to explode, use an oil bath for heating the still, and all operations should be carried out behind an adequate shield. [Kauer Org Synth Coll Vol IV 412 1963, Beilstein 3 III 233]. This reagent is useful in the Mitsunobu reaction [Mitsunobu Synthesis 1 1981, Gennari et al. J Am Chem Soc 108 6394 1986, Evans et al. J Am Chem Soc 108 6394 1986, Hughes Org React 42 335 1992, Dodge et al. Org Synth 73 110 1996, Hughes Org Prep Proc Int 28 127 1996, Ferguson & Marcelle J Am Chem Soc 128 4576 2006; see also di-tert-butyl azodicarboxylate and DEAD above].
Properties of Diisopropyl azodicarboxylate
| Melting point: | 3-5 °C |
| Boiling point: | 75 °C/0.25 mmHg (lit.) |
| Density | 1.027 g/mL at 25 °C (lit.) |
| vapor pressure | 1.04 hPa (20 °C) |
| refractive index | n |
| Flash point: | 223 °F |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Liquid |
| color | Clear to slightly turbid yellow-orange to red |
| Water Solubility | insoluble |
| Sensitive | Light Sensitive |
| BRN | 1912326 |
| CAS DataBase Reference | 2446-83-5(CAS DataBase Reference) |
Safety information for Diisopropyl azodicarboxylate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Diisopropyl azodicarboxylate
Diisopropyl azodicarboxylate manufacturer
RVR Labs Pvt Ltd
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![2,2'-AZOBIS[2-METHYL-N-(2-HYDROXYETHYL)PROPIONAMIDE]](https://img.chemicalbook.in/CAS/GIF/61551-69-7.gif)
You may like
-
 2446-83-5 99%View Details
2446-83-5 99%View Details
2446-83-5 -
 2446-83-5 98%View Details
2446-83-5 98%View Details
2446-83-5 -
 Diisopropyl azodicarboxylate CAS 2446-83-5View Details
Diisopropyl azodicarboxylate CAS 2446-83-5View Details
2446-83-5 -
 Diisopropyl azodicarboxylate CAS 2446-83-5View Details
Diisopropyl azodicarboxylate CAS 2446-83-5View Details
2446-83-5 -
 Diisopropyl Azodicarboxylate (40% in Toluene, ca. 1.9mol/L) CAS 2446-83-5View Details
Diisopropyl Azodicarboxylate (40% in Toluene, ca. 1.9mol/L) CAS 2446-83-5View Details
2446-83-5 -
 Diisopropyl azodicarboxylate; DIAD 98% CAS 2446-83-5View Details
Diisopropyl azodicarboxylate; DIAD 98% CAS 2446-83-5View Details
2446-83-5 -
 Diisopropyl AzodicarboxylateView Details
Diisopropyl AzodicarboxylateView Details
2446-83-5 -
 Diisopropyl Azodicarboxylate Cas 2446 83 5View Details
Diisopropyl Azodicarboxylate Cas 2446 83 5View Details
2446-83-5
