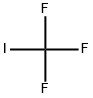
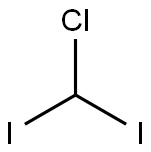
Diiodomethane
Synonym(s):Diiodomethane;Methylene iodide
- CAS NO.:75-11-6
- Empirical Formula: CH2I2
- Molecular Weight: 267.84
- MDL number: MFCD00001079
- EINECS: 200-841-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57

What is Diiodomethane?
Description
Diiodomethane (CH2I2) is an iodine containing organic compound. Its decomposition in acetonitrile initiated by 310nm light has been investigated. Femtosecond pump-probe spectroscopic and ab initio molecular dynamics simulations studies of the photodecomposition of CH2I2 suggest the formation of the isomer of diiodomethane (CH2I-I) as hot photoproduct. In the atmosphere, it undergoes photolysis in the presence of ozone to afford iodine oxide (IO) which results in the formation of aerosols. Its vacuum ultraviolet (VUV) photoabsorption spectrum has been reported.

Diiodomethane has been used as a probe liquid for evaluation of the polar and dispersive components of the surface energy of the catecholamine coated fiber surfaces. It may be used for the preparation of cyclopropyl ketones, esters and amides.
Diiodomethane may be used as a probe solvent in contact angle measurement to analyze the hydrophilicity of polymer surfaces.
https://www.sigmaaldrich.com/catalog/product/sial/158429
Chemical properties
Diiodomethane is a light yellow or gold liquid with chloroform-like odour. It has a relative density of 3.325 at 20°C and can be mixed with benzene to produce liquids with different densities. These mixtures are used in the determination of the refractive indices of minerals and for the separation of minerals according to density.
The Uses of Diiodomethane
Diiodomethane is used to determine the density of minerals and other solid samples due to its high density. It is used as an optical contact liquid to determine the refractive index of certain gemstones. In simmons-smith reaction, it acts as a reagent to generate methylene (carbine) free radical. It reacts with olefins to prepare cyclopropanes with high stereo specificity.
The Uses of Diiodomethane

To a solution of diethylzinc (1.0M in hexane, 46 mL, 46 mmol) in DCM (200 mL) was added dropwise a solution of TFA (3.54 mL, 46 mmol) in DCM (50 mL) at 0 C over 30 min. To the mixture was then added dropwise a solution of diiodomethane (3.7 mL, 46 mmol) in DCM (50 mL) at 0 C over 45 min. The mixture was stirred at 0 C for 1 h. A solution of the SM (2.52 g, 20 mmol) in DCM (30 mL) was added, and the reaction mixture was stirred at RT overnight. The mixture was quenched with sat aq NH4Cl (200 mL) and extracted with DCM. The org layer was dried (MgSO4) and concentrated in vacuo. The resulting material was purified by silica gel chromatography (20% EtOAc/hexane) to provide the product as a colorless oil. [1.77 g, 63%]
What are the applications of Application
Diiodomethane is a reagent for cyclopropanation in the Simmons-Smith reaction
What are the applications of Application
Diiodomethane is used as the dispersive (non-polar) liquid while de-ionized water and glycerol as polar liquids. It is also used in separating mixtures of minerals. In determining the specific gravity of minerals and other substances. In the manufacture of x-ray contrast media.
General Description
Diiodomethane (CH2I2) is an iodine containing organic compound. Its decomposition in acetonitrile initiated by 310nm light has been investigated. Femtosecond pump-probe spectroscopic and ab initio molecular dynamics simulations studies of the photodecomposition of CH2I2 suggest the formation of the isomer of diiodomethane (CH2I-I) as hot photoproduct. In the atmosphere, it undergoes photolysis in the presence of ozone to afford iodine oxide (IO) which results in the formation of aerosols. Its vacuum ultraviolet (VUV) photoabsorption spectrum has been reported.
Hazard
May be irritating and narcotic.
Diiodomethane has the advantages of high opacity,ease of penetration,and ease of removal because it evaporates fairly quickly.However,it can cause skin burns.
Synthesis
Diiodomethane can be prepared from the widely available solvent dichloromethane by the action of sodium iodide in acetone in the Finkelstein reaction:
CH2Cl2 + 2 NaI → CH2I2 + 2 NaCl.
It can also be prepared by reducing iodoform with elemental phosphorus or sodium arsenite:
CHI3 + Na3AsO3 + NaOH → CH2I2 + NaI + Na3AsO.
Diiodomethane is prepared by reacting iodoform with sodium acetate in ethanol.
Purification Methods
Fractionally distil it under reduced pressure, then fractionally crystallise it by partial freezing, and stabilize it with silver wool if necessary. It has also been purified by drying over CaCl2 and fractionally distilling from Cu powder. Store it in the dark. [Beilstein 1 IV 97.]
Properties of Diiodomethane
| Melting point: | 6 °C |
| Boiling point: | 67-69 °C11 mm Hg(lit.) |
| Density | 3.325 g/mL at 25 °C(lit.) |
| vapor density | 9.25 (vs air) |
| refractive index | 1.737 |
| Flash point: | 181°C |
| storage temp. | Store below +30°C. |
| solubility | 0.8g/l |
| form | Liquid |
| appearance | Colorless liquid |
| color | deep yellow |
| Specific Gravity | 3.325 |
| Water Solubility | 14 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,6066 |
| BRN | 1696892 |
| Dielectric constant | 5.3(-4℃) |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong bases. Reacts violently with alkali metal salts. May discolour on exposure to light. |
| CAS DataBase Reference | 75-11-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Methane, diiodo-(75-11-6) |
| EPA Substance Registry System | Methylene iodide (75-11-6) |
Safety information for Diiodomethane
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Diiodomethane
Diiodomethane manufacturer
JSK Chemicals
DeliCare LifeSciences Pvt Ltd
Svaks Biotech India Pvt Ltd
ARRAKIS INDUSTRIES LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 DIIODOMETHANE 98%View Details
DIIODOMETHANE 98%View Details -
 Diiodomethane 99%View Details
Diiodomethane 99%View Details -
 Diiodomethane CAS 75-11-6View Details
Diiodomethane CAS 75-11-6View Details
75-11-6 -
 Diiodomethane, 98% CAS 75-11-6View Details
Diiodomethane, 98% CAS 75-11-6View Details
75-11-6 -
 Diiodomethane (stabilized with Copper chip) CAS 75-11-6View Details
Diiodomethane (stabilized with Copper chip) CAS 75-11-6View Details
75-11-6 -
 METHYLENE IODIDE 99%View Details
METHYLENE IODIDE 99%View Details -
 Diiodomethane CASView Details
Diiodomethane CASView Details -
 Diiodomethane 98% CAS 75-11-6View Details
Diiodomethane 98% CAS 75-11-6View Details
75-11-6