DIETHYLALUMINUM CHLORIDE
Synonym(s):Chloro diethyl aluminum
- CAS NO.:96-10-6
- Empirical Formula: C4H10AlCl
- Molecular Weight: 120.56
- MDL number: MFCD00000459
- EINECS: 202-477-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
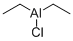
What is DIETHYLALUMINUM CHLORIDE?
Chemical properties
colorless solution
Chemical properties
The aluminum alkyl halides are flammable, reactive, and may be spontaneously combustible in air. They are colorless to yellow liquids. Ethylaluminum dichloride:(563-43-9):
Production Methods
Ethylaluminum sesquichloride (26.5 kg) added to a nitrogen-purged reactor, was heated to 175 ℃. Then, while the mixture was stirred vigorously, 1.1 kg sodium was added over a 30 min period; the mixture was further heated at 155 – 190 ℃ for 60 min. The diethylaluminum chloride product distilled from the reactor at 100 – 161 ℃ (1.3 – 6.1 kPa). In this example, an excess of ethylaluminum sesquichloride was employed to facilitate draining the voluminous byproduct salt and aluminum solids from the reactor. In an alternate approach, a heavy hydrocarbon oil, added prior to reaction, may be employed to remove the solids in slurry form, permitting the use of a stoichiometric ratio of ethylaluminum sesquichloride and sodium reactants.
General Description
Colorless liquid. Dangerous fire and explosion hazard. Used as an intermediate in production of organometallics.
Air & Water Reactions
Pyrophoric in air [Hawley]. Reacts violently with water, Rose(1961).
Reactivity Profile
Organometallics, such as DIETHYLALUMINUM CHLORIDE, are reactive with many other groups. Incompatible with acids and bases. Organometallics are good reducing agents and therefore incompatible with oxidizing agents. Often reactive with water to generate toxic or flammable gases. Organometallics containing halogens (fluorine, chlorine, bromine, iodine) bonded to the metal typically with generate gaseous hydrohalic acids (HF, HCl, HBr, HI) with water.
Potential Exposure
These materials are used as components of olefin polymerization catalysts. The reader is referred to the entry on “Aluminum alkyls” for additional information on this entry. The aluminum alkyl halides parallel very closely the aluminum alkyls
Shipping
UN3052 Spontaneously combustible. Water reactive releasing large quantities of toxic and deadly hydrogen gas. (Note: this number does not appear in the 49/CFR HazMat tables)
Purification Methods
Distil it from excess dry NaCl (to remove ethyl aluminium dichloride) in a 50-cm column containing a heated nichrome spiral. [Beilstein 4 IV 4403.]
Incompatibilities
The aluminum alkyl halides are strong reducing agents; they react—possibly violently—with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. These chemicals react violently with nitromethaneEthylaluminum sesquichloride reacts explosively with carbon tetrachloride at room temperature. This chemical reacts violently with water, forming corrosive hydrogen chloride and flammable ethane gas. Diethylaluminum chloride may form an explosive product with chlorine azide.
Properties of DIETHYLALUMINUM CHLORIDE
| Melting point: | -85°C |
| Boiling point: | 125°C 50mm |
| Density | 0.887 g/mL at 25 °C |
| vapor pressure | 3 mmHg ( 60 °C) |
| Flash point: | −9 °F |
| storage temp. | 0-6°C |
| solubility | Miscible with hexane. |
| form | Solution |
| color | Colorless |
| Specific Gravity | 0.711 (20/4℃) |
| Water Solubility | reac H2O [CRC10] |
| Sensitive | Air & Moisture Sensitive |
| Merck | 326 |
| BRN | 4123259 |
| CAS DataBase Reference | 96-10-6(CAS DataBase Reference) |
| EPA Substance Registry System | Aluminum, chlorodiethyl- (96-10-6) |
Safety information for DIETHYLALUMINUM CHLORIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H250:Pyrophoric liquids; Pyrorophoric solids H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H304:Aspiration hazard H314:Skin corrosion/irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H373:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for DIETHYLALUMINUM CHLORIDE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
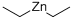

You may like
-
 Diethylaluminium chloride, 1M solution in Hexanes CAS 96-10-6View Details
Diethylaluminium chloride, 1M solution in Hexanes CAS 96-10-6View Details
96-10-6 -
![DEAC [NEAT] CAS 96-10-6](https://img.chemicalbook.in//Content/image/CP5.jpg) DEAC [NEAT] CAS 96-10-6View Details
DEAC [NEAT] CAS 96-10-6View Details
96-10-6 -
 Diethyl Aluminium Chloride 1.0M in Heptane CAS 96-10-6View Details
Diethyl Aluminium Chloride 1.0M in Heptane CAS 96-10-6View Details
96-10-6 -
 Diethyl Aluminium Cyanide 25% in Toluene CAS 96-10-6View Details
Diethyl Aluminium Cyanide 25% in Toluene CAS 96-10-6View Details
96-10-6 -
 Diethyl Aluminium Chloride 25% in Heptane CAS 96-10-6View Details
Diethyl Aluminium Chloride 25% in Heptane CAS 96-10-6View Details
96-10-6 -
 Diethyl Aluminium Chloride 1.0M in Hexane CAS 96-10-6View Details
Diethyl Aluminium Chloride 1.0M in Hexane CAS 96-10-6View Details
96-10-6 -
 Diethyl Aluminium Cyanide 1.0M in Toluene CAS 96-10-6View Details
Diethyl Aluminium Cyanide 1.0M in Toluene CAS 96-10-6View Details
96-10-6 -
 Diethyl aluminium chloride 1M in Hexane CAS 96-10-6View Details
Diethyl aluminium chloride 1M in Hexane CAS 96-10-6View Details
96-10-6
