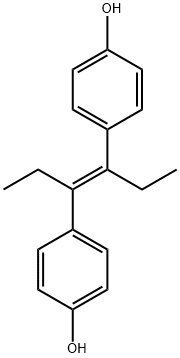
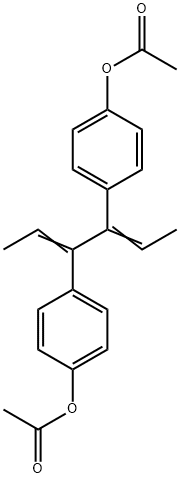
DIENESTROL
Synonym(s):3,4-Bis(4-hydroxyphenyl)-2,4-hexadiene
- CAS NO.:84-17-3
- Empirical Formula: C18H18O2
- Molecular Weight: 266.33
- MDL number: MFCD00050983
- EINECS: 201-519-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
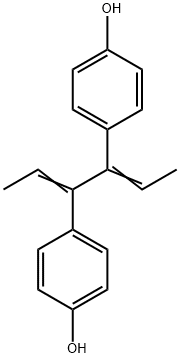
What is DIENESTROL?
Chemical properties
Colorless, needles or powder; odorless. Soluble in alcohol; practically insoluble in water; sensitive to light.
Originator
Synestrol,Schering,US,1947
The Uses of DIENESTROL
Dienestrol is a metabolite of Diethylstilbestrol, a non-steroidal synthetic estrogen derivative.
The Uses of DIENESTROL
Medicine (estrogenic hormone).
What are the applications of Application
Dienestrol is A non-steroidal estrogen structurally related to stilbestrol
Definition
ChEBI: An olefinic compound that is hexa-2,4-diene substituted by 4-hydroxyphenyl groups at positions 3 and 4 respectively.
Manufacturing Process
Preparation of γ,δ-Bis-(4-Hydroxylphenyl)-Hexane-γ,δ-Diol: A sodium amalgam is prepared containing 6 grams of sodium and 400 grams of mercury. The
amalgam is covered with a solution of 20 grams of 4-hydroxypropiophenone in
a mixture of 30 ml of 5 N sodium hydroxide solution and 220 ml of water and
the mixture is heated to 28°-30°C and stirred gently. The reduction is
accompanied by development of heat and the temperature of the solution
rises to 34°-35°C, and then falls slowly. After 5 hours the alkaline solution is
separated from the mercury and diluted with 3 or 4 times its volume of water,
when, in order to form the benzoyl derivatives of the products, the solution is
vigorously stirred, while it is being cooled, with 20 ml of benzoyl chloride, the
solution being kept at a temperature of 15°-20°C. When the reaction is
completed, the benzoyl derivatives are filtered off, washed with water and
recrystallized from a mixture of benzene and alcohol, when a product with a
melting point of 195°-215°C is obtained.
Preparation of Dienestrol: In order to obtain dienoestrol, 14.6 grams of dry
4,4'-dibenzoate are refluxed with a mixture of 40 ml of acetic anhydride and
40 ml acetylchloride by heating in an oil-bath at about 90°C for 6 hours after
which the bath temperature is increased to 120°C and heating continued for a
further 18 hours, after which time the evolution of hydrogen chloride
practically ceases. The mixture is allowed to cool for several hours and the
crystals which separate are filtered off and recrystallized from an alcoholbenzene mixture when the product melts at 210°-222°C. This product is
converted into dienoestrol by adding 10.8 grams of it to 100 ml of 10% (w/v)
alcoholic potassium hydroxide solution and then refluxing during 1 hour. After
dilution with 200 ml of water and filtration from a small amount of insoluble
material, dienoestrol is precipitated from the alkaline solution by treatment
with carbon dioxide. It is filtered off, washed with water and recrystallized
from dilute alcohol after which it melts at 233°-234°C according to US Patent
2,464,203.
brand name
Avc/dienestrol;Crinohermal fem;D.v.;Dienostrol cream;Dienstrogen;Dinol;Dufemine;Foragynol;Frein;Klianyi forte;Klianyl;Neo-oestrogenine;Ortho (cream);Ortho dienestrol cream.
Therapeutic Function
Estrogen
World Health Organization (WHO)
Dienestrol is a stilbene derivative. See WHO comment for diethylstilbestrol. Vaginal forms of dienestrol, which were introduced in 1947, are currently available in over 35 countries for the management of hypoestrogenic vaginal atrophy. (Reference: (WHODI) WHO Drug Information, 77.1, 16, 1977)
Safety Profile
Suspected human carcinogen. Human mutation data reported. Experimental reproductive effects. Used as a drug for the treatment of postmenopausal symptoms. When heated to decomposition it emits acrid smoke and irritating fumes.
Properties of DIENESTROL
| Melting point: | 229 °C |
| Boiling point: | 349.54°C (rough estimate) |
| Density | 1.184 g/cm3 |
| refractive index | 1.4800 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | neat |
| pka | 9.21±0.15(Predicted) |
| form | Solid |
| color | Pale Yellow to Pale Beige |
| Water Solubility | 3mg/L(37 ºC) |
| Merck | 3104 |
| BRN | 2053694 |
| CAS DataBase Reference | 84-17-3 |
| EPA Substance Registry System | Dienestrol (84-17-3) |
Safety information for DIENESTROL
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H350:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for DIENESTROL
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Dienestrol CAS 84-17-3View Details
Dienestrol CAS 84-17-3View Details
84-17-3 -
 Dienestrol CAS 84-17-3View Details
Dienestrol CAS 84-17-3View Details
84-17-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1