DIENESTROL
- CAS NO.:13029-44-2
- Empirical Formula: C18H18O2
- Molecular Weight: 266.33
- MDL number: MFCD00050983
- EINECS: 201-519-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:43:48
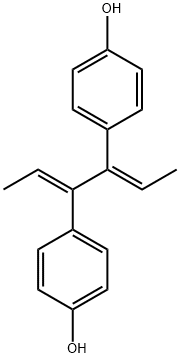
What is DIENESTROL?
Absorption
Systemic absorption and mode of action of dienestrol are undetermined.Estrogens diffuse into their target cells and interact with a protein receptor. Target cells include the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary. Estrogens increase the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid-binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) from the anterior pituitary. The combination of an estrogen with a progestin suppresses the hypothalamic-pituitary system, decreasing the secretion of gonadotropin-releasing hormone (GnRH).
Toxicity
Symptoms of overdose include nausea and vomiting, and withdrawal bleeding may occur in females.
Chemical properties
Off-White Solid
The Uses of DIENESTROL
A metabolite of Diethylstilbestrol
The Uses of DIENESTROL
A metabolite of Diethylstilbestrol.
Indications
For use in the treatment of atrophic vaginitis and kraurosis vulvae.
Background
Dienestrol is a synthetic, non-steroidal estrogen. It is an estrogen receptor agonist. Estrogens work partly by increasing a normal clear discharge from the vagina and making the vulva and urethra healthy. Using or applying an estrogen relieves or lessens: dryness and soreness in the vagina, itching, redness, or soreness of the vulva. Conditions that are treated with vaginal estrogens include a genital skin condition (vulvar atrophy), inflammation of the vagina (atrophic vaginitis), and inflammation of the urethra (atrophic urethritis).
What are the applications of Application
E,E-Dienestrol is a metabolite of Diethylstilbestrol
Definition
ChEBI: Dienestrol is an olefinic compound that is hexa-2,4-diene substituted by 4-hydroxyphenyl groups at positions 3 and 4 respectively. It has a role as a xenoestrogen. It is a member of phenols and an olefinic compound.
Pharmacokinetics
Estrogens diffuse into their target cells and interact with a protein receptor. Target cells include the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary. Estrogens increase the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid-binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) from the anterior pituitary. The combination of an estrogen with a progestin suppresses the hypothalamic-pituitary system, decreasing the secretion of gonadotropin-releasing hormone (GnRH).
Metabolism
Hepatic.
Properties of DIENESTROL
| Melting point: | 224-226°C |
| Boiling point: | 349.54°C (rough estimate) |
| Density | 1.1305 (rough estimate) |
| refractive index | 1.4800 (estimate) |
| storage temp. | Amber Vial, -20°C Freezer, Under Inert Atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 10.18±0.15(Predicted) |
| color | Pale Beige |
| Stability: | Light sensitive |
Safety information for DIENESTROL
Computed Descriptors for DIENESTROL
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


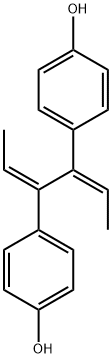
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1