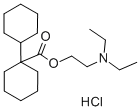
DICYCLOMINE HYDROCHLORIDE
Synonym(s):[1,1-Bicyclohexyl]-1-carboxylic acid 2-(diethylamino)ethyl ester hydrochloride;2-(Diethylamino)ethyl 1-cyclohexylcyclohexane-1-carboxylate hydrochloride
- CAS NO.:67-92-5
- Empirical Formula: C19H36ClNO2
- Molecular Weight: 345.95
- MDL number: MFCD00079158
- EINECS: 200-671-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
What is DICYCLOMINE HYDROCHLORIDE?
Description
Dicyclomine is an M1 and M2 muscarinic acetylcholine receptor antagonist (Kis = 3.16 and 44.7 nM, respectively). It inhibits inositol phosphate accumulation induced by the non-selective acetylcholine agonist carbamoylcholine (carbachol; ) in guinea pig cortex (Ki = 12 nM; pA2 = 7.88). Dicyclomine (10 mg/kg, i.v.) inhibits potassium-induced contraction of ileum, colon, and duodenum in anesthetized rats by 21.85, 30.81, and 37.86%, respectively. Formulations containing dicyclomine have been used to treat functional and irritable bowel syndrome and to relieve muscle spasms in the gastrointestinal tract.
Chemical properties
White Crystalline Powder
Originator
Bentyl ,Merrell National ,US,1950
The Uses of DICYCLOMINE HYDROCHLORIDE
Dicyclomine Hydrochloride can be used as medication to treat gastric diseases.
The Uses of DICYCLOMINE HYDROCHLORIDE
Used as a gastrointestinal antispasmodic antacid
The Uses of DICYCLOMINE HYDROCHLORIDE
anticholinergic
Definition
ChEBI: The hydrochloride salt of dicyclomine. An anticholinergic, it is used to treat or prevent spasm in the muscles of the gastrointestinal tract, particularly that associated with irritable bowel syndrome.
Manufacturing Process
155 grams of 1-phenylcyclohexanecyanide, 350 cc of concentrated sulfuric
acid and 1,130 cc of ethyl alcohol are refluxed vigorously for 48 hours. The
remaining alcohol is then removed by vacuum distillation and the residue is
poured into 1 liter of ice water. An oil separates which is extracted 3 times
with 200 cc portions of petroleum ether, the extracts are combined and heated on a steam bath to remove the ether. The resulting crude ester may
be used directly for the reesterification operation or it may be distilled to
purify it first. A mixture of the ester so obtained, 155 grams of βdiethylaminoethanol and 800 cc of dry xylene are placed in a reaction vessel
with about 2 grams of sodium. The vessel is heated in an oil bath at 150°-
160°C. A xylene-ethanol azeotrope distills over at about 78°-82°C over a
period of 2 to 3 hours. The distillate is cooled and shaken with about 3 times
its volume of water, the decrease in volume of the distillate being considered a
measure of the amount of alcohol formed. When 80-90% of the theoretical
amount of alcohol is obtained in the distillate the reaction mixture is subjected
to vacuum distillation to remove most of the xylene and unreacted
diethylaminoethanol. The residue is poured into 500 cc of benzene which is
then extracted 3 times with 500 cc portions of water.
The washed benzene layer is diluted with an equal volume of ether and
alcoholic hydrochloric acid is added until the mixture is acid to Congo red. A
white crystalline solid forms which is dissolved in 300-400 cc of alcohol and
diluted with ether to the point where precipitation starts. A few drops of
butanone are added, the solution is cooled to -10°C, and filtered to recover
the crystals which separate. The product is obtained in the form of white
needles melting at 159°-160°C, in good yield.
13 parts of β-diethylaminoethyl 1-phenylcyclohexanecarboxylate
hydrochloride, 125 parts of glacial acetic acid and 0.3 part of Adams' catalyst
are heated to 70°C and shaken with hydrogen at 50 lb pressure until 90-
100% of the theoretical hydrogen is absorbed. The acetic acid is then
removed by distillation and the residue recrystallized from butanone, giving
the above product as a crystalline hydrochloride melting at 165°-166°C, in
good yields. This product may also be prepared by reacting cyclohexyl
bromide with cyclohexyl cyanide with the use of sodium amide followed by
alcoholysis and reesterification.
brand name
Bentyl (Axcan Scandipharm).
Therapeutic Function
Spasmolytic
General Description
Dicyclomine hydrochloride,2-(diethylamino)ethyl bicyclohexyl-1-carboxylatehydrochloride (Bentyl), has some muscarinic receptorsubtype selectivity. It binds more firmly to M1 and M3 thanto M2 and M4 receptors.76Dicyclomine hydrochloride has one eighth of the neurotropicactivity of atropine and approximately twice themusculotropic activity of papaverine. This preparation, firstintroduced in 1950, has minimized the adverse effects associatedwith the atropine-type compounds.
Clinical Use
Dicyclomine Hydrochloride is used for itsspasmolytic effect on various smooth muscle spasms, particularlythose associated with the GI tract. It is also usefulin dysmenorrhea, pylorospasm, and biliary dysfunction.
Safety Profile
Poison by intravenous route.Moderately toxic by ingestion. Human systemic effects byingestion: rigidity, dyspnea, cyanosis. When heated todecomposition it emits very toxic fumes of HCl and NOx.
Properties of DICYCLOMINE HYDROCHLORIDE
| Melting point: | 164-166°C |
| storage temp. | -20°C Freezer |
| solubility | H2O: 50 mg/mL |
| form | powder |
| color | off-white |
| EPA Substance Registry System | 2-Diethylaminoethyl bicyclohexyl-1-carboxylate hydrochloride (67-92-5) |
Safety information for DICYCLOMINE HYDROCHLORIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DICYCLOMINE HYDROCHLORIDE
DICYCLOMINE HYDROCHLORIDE manufacturer
Chemoil Pharma
Festiva Pharma
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![[1,1'-bicyclohexyl]-1-carboxylic acid](https://img.chemicalbook.in/CAS/GIF/60263-54-9.gif)
You may like
-
 67-92-5 DICYCLOMINE HCl IP 99%View Details
67-92-5 DICYCLOMINE HCl IP 99%View Details
67-92-5 -
 67-92-5 99%View Details
67-92-5 99%View Details
67-92-5 -
 Dicyclomine hydrochloride 98%View Details
Dicyclomine hydrochloride 98%View Details
67-92-5 -
 67-92-5 Dicyclomine hydrochloride 98%View Details
67-92-5 Dicyclomine hydrochloride 98%View Details
67-92-5 -
 Dicyclomine hydrochloride 98%View Details
Dicyclomine hydrochloride 98%View Details
67-92-5 -
 Dicyclomine Hydrochloride 99%View Details
Dicyclomine Hydrochloride 99%View Details -
 67-92-5 DICYCLOMINE HCl 95-99 %View Details
67-92-5 DICYCLOMINE HCl 95-99 %View Details
67-92-5 -
 Dicyclomine hydrochloride CAS 67-92-5View Details
Dicyclomine hydrochloride CAS 67-92-5View Details
67-92-5
