Dapoxetine hydrochloride
- CAS NO.:129938-20-1
- Empirical Formula: C21H24ClNO
- Molecular Weight: 341.88
- MDL number: MFCD08272809
- EINECS: 640-411-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-15 21:51:00
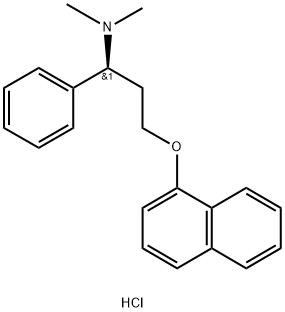
What is Dapoxetine hydrochloride?
Description
Dapoxetine hydrochloride, belonging to the class of SSRIs, was the first drug originally approved for the on-demand treatment of men with PE by seven European countries in 2008.
Premature ejaculation (PE) is the most common male sexual dysfunction. Dapoxetine is a short-acting SSRI. It is differentiated from the existing SSRI treatments for PE by the fact that it can be administered on an as-needed basis. In healthy subjects, dapoxetine is rapidly absorbed after oral administration with a peak plasma concentration (Tmax) occurring between 1.4 and 2h.
Chemical properties
Dapoxetine hydrochloride is a white to slightly yellow powder. It is freely soluble in methanol, propylene glycol, some organic solvents (eg. N,N-dimethylformamide) , slightly soluble in ethanol and almost insoluble in water. It is a BCSI class II compound, and poor solubility is a key factor affecting the difference in clinical efficacy. Dapoxetine hydrochloride exists in various crystal forms, the solubility of different crystal forms is quite different, and there is a phenomenon of phase and transformation between crystal forms.
Originator
Lilly (US)
The Uses of Dapoxetine hydrochloride
Dapoxetine hydrochloride is a short-acting novel selective serotonin reuptake inhibitor marketed for the treatment of premature ejaculation in men. Premature ejaculation (PE) is the most common male sexual disorder, estimated to affect up to 30% of men.
What are the applications of Application
Dapoxetine Hydrochloride is a selective serotonin reuptake inhibitor
brand name
Priligy
General Description
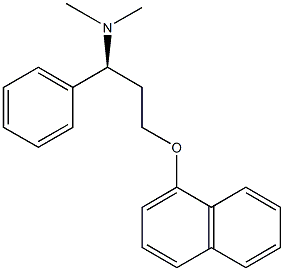
Dapoxetine ((+)-(S)-N,N-dimethyl-(α)-[2(1naphthal enyloxy)ethyl]-benzenemethanamine hydrochloride) possess a similar structure as that of fluoxetine.
Biochem/physiol Actions
Potent Selective serotonin reuptake inhibitor (SSRI); used in treatment of premature ejaculation
Synthesis
Dapoxetine can be synthesized in four chemical steps starting from R-1phenyl- 1,3-propanediol via selective tosylation of the primary hydroxy group with p-toluenesulfonyl chloride, triethylamine and 4-(dimethylamino) pyridine (DMAP), and subsequent condensation with 1naphthol by means of sodium- or lithium hydroxide to yield R-3-(1naphthyloxy)- 1-phenylpropanol as the key intermediate. Conversion of the alcohol group to the corresponding mesylate with methanesulfonyl chloride, triethylamine, and DMAP, followed by treatment with dimethylamine affords dapoxetine, which is then acidified to its hydrochloride salt.
storage
room temperature (desiccate)
Mode of action
The mechanism of action of dapoxetine in premature ejaculation is presumed to be linked to the inhibition of neuronal reuptake of serotonin and the subsequent potentiation of the neurotransmitter's action at pre- and post-synaptic receptors. Dapoxetine is a centrally-acting SSRI that modulates serotonin levels in relevant areas such as the lateral paragigantocellular nucleus through inhibition of the serotonin transporter (SERT). This compound also decreases peak amplitude and accelerates the decay rate of current inactivation in a variety of voltage-gated K+ channels.
Properties of Dapoxetine hydrochloride
| Melting point: | 175-1790C |
| alpha | D +135.78° (c = 2.18 in methanol) |
| storage temp. | room temp |
| solubility | DMSO: ≥20mg/mL |
| form | powder |
| color | white |
| optical activity | [α]/D +125 to +135°, c = 1 in methanol |
| Merck | 14,2821 |
| InChI | InChI=1/C21H23NO.ClH/c1-22(2)20(18-10-4-3-5-11-18)15-16-23-21-14-8-12-17-9-6-7-13-19(17)21;/h3-14,20H,15-16H2,1-2H3;1H/t20-;/s3 |
| CAS DataBase Reference | 129938-20-1(CAS DataBase Reference) |
Safety information for Dapoxetine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dapoxetine hydrochloride
| InChIKey | IHWDIQRWYNMKFM-OZYVVJTJNA-N |
| SMILES | O(C1=CC=CC2C=CC=CC1=2)CC[C@@H](C1C=CC=CC=1)N(C)C.Cl |&1:13,r| |
Dapoxetine hydrochloride manufacturer
Besil Chem LLP
New Products
DL-Lactide L-Lactide PLGA 50:50 – PEG, DLG501P1 ,M.W:PLGA (Mw) : ~10,000 da, PEG (Mw) : ~1500 da Poly(DL-Lactide)-co-Glycolide, DLG852A, M.W:7,000 - 17,000 da, I.V: 0.16 - 0.24 dL/g Polycaprolactone, CL10015 , M.W: 190,000 – 240,000 da , I.V: 1.30 – 1.70 dL/g Lawesson’s Reagent methyl vinyl sulfone Pyridine, 3-[4-(hexyloxy)-1,2,5-thiadiazol-3-yl]-1,2,5,6-tetrahydro-1-methyl-, (2E)-2-butenedioate (1:1) 5-NITRONICOTINIC ACID 3-Pyridineacetonitrile, α-amino-, hydrochloride (1:1) TETRAHYDRO-2-(2-PROPYNYLOXY)-2H-PYRAN (1-Cyano-cyclobutyl)-carbamic acid tert-butyl ester ETHYL 2-[(3-FORMYL-4-ISOPROPOXYPHENYL]-4- METHY-1,3-THIAZOLE-5-CARBOXYLATE ROPIVACAINE USP RELATED COMPOUND B (HCL SALT) OPEN RING DESULFATED AZTREONAM (E-ISOMER) / AZTREONAM USP RELATED COMPOUND B HYOSCYAMINE RELATED COMPOUND A HYDROCHLORIDE ASCORBIC ACID IMPURITY D ((3S,4R,5S)-METHYL 3,4,5,6-TETRAHYDROXY-2-OXOHEXANOATE) LUMEFANTRINE N-OXIDE (2 BTL) Linagliptin (S)-(+)-N-(2,3-Epoxypropyl)phthalimide or (S)-N-Glycidylphthalimide 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole 4-(2-chloroethyl)-α,α-dimethyl-) Benzeneacetic acid 4-Nitrophenyl Chloroformate 2-(4-Bromophenyl)-2-methylpropanoic acidRelated products of tetrahydrofuran



You may like
-
 129938-20-1 Dapoxetine HCL-IHS 98%View Details
129938-20-1 Dapoxetine HCL-IHS 98%View Details
129938-20-1 -
 Dapoxetine HCL 99%View Details
Dapoxetine HCL 99%View Details -
 Dapoxetine HCl 99% (HPLC) CAS 129938-20-1View Details
Dapoxetine HCl 99% (HPLC) CAS 129938-20-1View Details
129938-20-1 -
 Dapoxetine Hcl 99%View Details
Dapoxetine Hcl 99%View Details -
 Dapoxetine HCl 95-99%View Details
Dapoxetine HCl 95-99%View Details
129938-20-1 -
 Dapoxetine Hydrochloride CAS 129938-20-1View Details
Dapoxetine Hydrochloride CAS 129938-20-1View Details
129938-20-1 -
 Dapoxetine HCl 95.00% CAS 129938-20-1View Details
Dapoxetine HCl 95.00% CAS 129938-20-1View Details
129938-20-1 -
 Dapoxetine HCl 98% (HPLC) CAS 129938-20-1View Details
Dapoxetine HCl 98% (HPLC) CAS 129938-20-1View Details
129938-20-1
