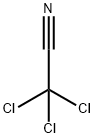
Dichloroacetonitrile
Synonym(s):2,2-Dichloroacetonitrile;Dichloromethyl cyanide
- CAS NO.:3018-12-0
- Empirical Formula: C2HCl2N
- Molecular Weight: 109.94
- MDL number: MFCD00040886
- EINECS: 221-159-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
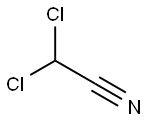
What is Dichloroacetonitrile?
Chemical properties
colourless liquid
The Uses of Dichloroacetonitrile
Dichloroacetonitrile can be used as a reactant to prepare:
- Chiral α, α-dichloro-β-aminonitriles via Pd-catalyzed enantioselective Mannich-type reaction with imines.
- α, α-dialkyl-substituted nitriles by an alkylation reaction with trialkylboranes in the presence of phenoxide base as a base.
- Halogenated pyridines via copper-catalyzed reaction with methacrolein.
- α,α-dichloro-β-hydroxy nitriles by condensation reaction with aldehydes and ketones in the presence of an alkoxide base.
- Selenium heterocycle derivatives via Diels–Alder cyclization with selenoaldehydes.
- Dichloroacetonitrile can also be used to develop an efficient method for the extraction and determination of common volatile halogenated disinfection by-products using the static headspace technique coupled with gas chromatography-mass spectrometry.
Definition
ChEBI: Dichloroacetonitrile is an aliphatic nitrile.
General Description
Clear liquid.
Air & Water Reactions
Burns slowly, emitting a thick black smoke, but will not flash . Water soluble.
Reactivity Profile
Dichloroacetonitrile is a halogenated nitrile. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition Dichloroacetonitrile emits toxic fumes of chlorine, cyanides and nitrogen oxides.
Fire Hazard
Dichloroacetonitrile is probably combustible.
Biochem/physiol Actions
Dichloroacetonitrile is direct-acting mutagen and induces DNA strand breaks in cultured human lymphoblastic cells. It induces apoptosis or necrosis in murine macrophage cell line via reactive oxygen intermediates-mediated oxidative mechanisms of cellular damage.
Purification Methods
Purify the nitrile by distillation or by gas chromatography. [Beilstein 2 IV 506.] FLAMMABLE.
Properties of Dichloroacetonitrile
| Boiling point: | 110-112 °C(lit.) |
| Density | 1.369 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 96 °F |
| storage temp. | 0-6°C
|
| solubility | soluble in Methanol |
| form | neat |
| color | Colorless to Light yellow to Light orange |
| Specific Gravity | 1.37 |
| BRN | 1739029 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 3018-12-0(CAS DataBase Reference) |
| IARC | 3 (Vol. 52, 71) 1999 |
| NIST Chemistry Reference | Acetonitrile, dichloro-(3018-12-0) |
| EPA Substance Registry System | Dichloroacetonitrile (3018-12-0) |
Safety information for Dichloroacetonitrile
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dichloroacetonitrile
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Dichloroacetonitrile CAS 3018-12-0View Details
Dichloroacetonitrile CAS 3018-12-0View Details
3018-12-0 -
 Dichloroacetonitrile CAS 3018-12-0View Details
Dichloroacetonitrile CAS 3018-12-0View Details
3018-12-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
