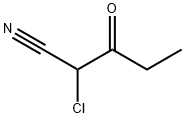
Chloroacetonitrile
Synonym(s):Chloroacetonitrile;Chloroethane nitrile
- CAS NO.:107-14-2
- Empirical Formula: C2H2ClN
- Molecular Weight: 75.5
- MDL number: MFCD00001885
- EINECS: 203-467-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13

What is Chloroacetonitrile?
Chemical properties
A clear, colorless liquid. Pungent odor. Chloroacetonitrile [107-14-2], chloromethanenitrile, chloromethyl cyanide, ClCH2CN, Mr 75.50, bp 124 – 126℃, d204 1.1896, n20D 1.426, is a colorless liquid with pungent odor. It is miscible with hydrocarbons and ethanol; immiscible with water. Chloroacetonitrile can be selectively obtained by the photochemical chlorination of acetonitrile with chlorine in the presence of, for example, SnCl4. Another method is based on the dehydration of chloroacetamide with, e.g., phosphorous pentoxide. Chloroacetonitrile is used as an organic intermediate, e.g., in the synthesis of the cardiovascular drug guanethidine [36] and the insecticide fenoxycarb.
The Uses of Chloroacetonitrile
Chloroacetonitrile is used in the electrochemical synthesis of cyanoacetic acid with carbon dioxide. It is involved in phase-transfer-catalyzed Darzen's condensation reaction with cyclohexanone. It is also used as an eluent additive in thermospray liquid chromatography/mass spectrometry. Further, it is used to prepare polysubstituted pyrido[1,2-a]benzimidazole by reacting with other reactant such as malononitrile, aromatic aldehyde and pyridine.
The Uses of Chloroacetonitrile
Fumigant, intermediate.
Preparation
In a 3-L round-bottomed, threenecked flask fitted with an efficient mechanical stirrer, a reflux condenser, and a thermometer were placed phosphorus pentoxide (170 g, 1.2 mol), chloroacetamide 1423 (187 g, 2 mol), and dry technical grade trimethylbenzene (800 mL). The mixture was gently refluxed with vigorous stirring for 1 h. It was then allowed to cool to about 100 C° with continuous stirring, and the reflux condenser was replaced with a distillation head fitted with a thermometer and a water-cooled condenser. The crude product and part of the solvent were distilled at atmospheric pressure. The yield of crude product boiling at 124–128 C° was 121–131 g (80–87%). In order to obtain a pure product, the crude chloroacetonitrile was mixed with phosphorus pentoxide (10 g) and redistilled through an efficient packed fractionating column. The yield of pure chloroacetonitrile distilling at 123–124 C° was 93–106 g (62–70%).
Definition
ChEBI: Chloroacetonitrile is a nitrile.
General Description
A colorless liquid with a pungent odor. Flash point 118°F. Insoluble in water and denser than water. Hence, sinks in water. Very toxic by ingestion, inhalation and skin absorption. A lachrymator. Used to make other chemicals and as a fumigant.
Air & Water Reactions
Flammable. Insoluble in water and denser than water. Hence, sinks in water. Reacts with water and steam to produce toxic vapors of hydrogen chloride.
Reactivity Profile
Chloroacetonitrile reacts with water, steam, strong acids or acid fumes to produce toxic vapors of hydrogen chloride. When heated to decomposition, Chloroacetonitrile emits highly toxic fumes of hydrogen cyanide and hydrogen chloride [Sax, 2nd ed., 1963, p. 600].
Hazard
Irritant. Questionable carcinogen.
Health Hazard
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Inhalation or contact with some of these materials will irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion and poison hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Poison by ingestion, skin contact, and intraperitoneal routes. Moderately toxic by inhalation. A skin irritant. Human mutation data reported. Questionable carcinogen with experimental tumorigenic data. Flammable liquid. See also NITRILES. When heated to decomposition it emits very toxic fumes of Cl-, NOx, and CN-.
Potential Exposure
A chlorinated haloacetonitrile used as a fumigant and as a manufacturing chemical intermediate for making other chemicals
Shipping
UN2668 Chloroaceto nitrile Hazard class: 6.1, Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid Inhalation Hazard Zone B.
Purification Methods
Reflux it with P2O5 for one day, then distil it through a helices-packed column. Also purified by gas chromatography. [Beilstein 2 IV 492.] LACHRYMATOR, HIGHLY TOXIC.
Incompatibilities
Highly flammable, forms explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Reacts with water and steam, releasing toxic and corrosive vapors of hydrogen chloride. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously low aqueous solubility. They are also insoluble in aqueous acids. with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Chloroacetonitrile
| Melting point: | 38℃ |
| Boiling point: | 124-126 °C (lit.) |
| Density | 1.193 g/mL at 25 °C (lit.) |
| vapor density | 3 (vs air) |
| vapor pressure | 1.78 psi ( 20 °C) |
| refractive index | n |
| Flash point: | 118 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform, Ethyl Acetate |
| form | Liquid |
| color | Clear colorless |
| Water Solubility | INSOLUBLE |
| BRN | 506028 |
| Exposure limits | NIOSH: IDLH 14 ppm(25 mg/m3) |
| Dielectric constant | 30.0 |
| Stability: | Stable, but reacts with water. Combustible. Incompatible with water, moisture, strong oxidizing agents, acids. |
| CAS DataBase Reference | 107-14-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetonitrile, chloro-(107-14-2) |
| IARC | 3 (Vol. 52, 71) 1999 |
| EPA Substance Registry System | Chloroacetonitrile (107-14-2) |
Safety information for Chloroacetonitrile
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H310:Acute toxicity,dermal H319:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Chloroacetonitrile
| InChIKey | RENMDAKOXSCIGH-UHFFFAOYSA-N |
Chloroacetonitrile manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
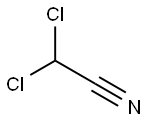


![2-CHLORO-3-[2,6-DICHLORO-4-(TRIFLUOROMETHYL)PHENYL]PROPANENITRILE](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB9216026.gif)
You may like
-
 CHLORO ACETON ITRILE 99%View Details
CHLORO ACETON ITRILE 99%View Details -
 107-14-2 Chloroacetonitrile 98%View Details
107-14-2 Chloroacetonitrile 98%View Details
107-14-2 -
 107-14-2 Chloroacetonitrile 98%View Details
107-14-2 Chloroacetonitrile 98%View Details
107-14-2 -
 Chloroacetonitrile 107-14-2 99.31View Details
Chloroacetonitrile 107-14-2 99.31View Details
107-14-2 -
 Chloroacetonitrile, 98+% CAS 107-14-2View Details
Chloroacetonitrile, 98+% CAS 107-14-2View Details
107-14-2 -
 Chloroacetonitrile CAS 107-14-2View Details
Chloroacetonitrile CAS 107-14-2View Details
107-14-2 -
 Chloroacetonitrile,LR 98% CAS 107-14-2View Details
Chloroacetonitrile,LR 98% CAS 107-14-2View Details
107-14-2 -
 ChloroacetonitrileView Details
ChloroacetonitrileView Details
107-14-2
