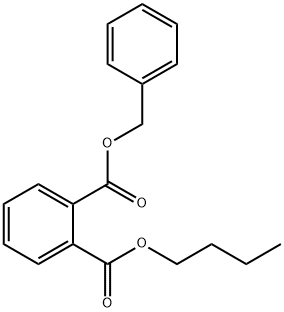
DI-N-OCTYL PHTHALATE
Synonym(s):DNOP;Phthalic acid di-n-octyl ester
- CAS NO.:117-84-0
- Empirical Formula: C24H38O4
- Molecular Weight: 390.56
- MDL number: MFCD00015292
- EINECS: 204-214-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
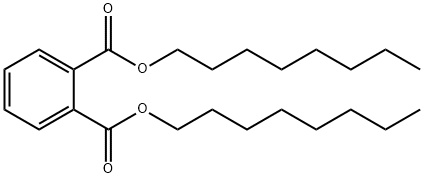
What is DI-N-OCTYL PHTHALATE?
Description
DNOP is a liquid. Molecular weight= 390.62;Boiling point = 230℃ under 5 mmHg; Flash point = 215℃;Autoignition temperature= 390℃. Explosive Limits:LEL = 0.3% at 245℃; UEL—unknown. HazardIdentification (based on NFPA-704 M Rating System): Health2, Flammability 1, Reactivity 0. Insoluble in water.
Chemical properties
Colorless liquid
Physical properties
Clear, light colored, viscous, oily liquid with a slight odor
The Uses of DI-N-OCTYL PHTHALATE
Di-n-octyl phthalate is used as a plasticizer for many resins and elastomers. It acts as an additive. It is also used for medical tubing and blood storage bags, wire and cables, carpet back coating, floor tile and in cosmetics.
The Uses of DI-N-OCTYL PHTHALATE
Phthalate ester used as plasticizers and additives. Used in toxicology studies as well as risk assessment studies of food contamination that occurs via migration of phthalates into foodstuffs from food-contact materials (FCM).
The Uses of DI-N-OCTYL PHTHALATE
Dioctyl phthalate is used as a plasticizer in various plastic materials.
Definition
ChEBI: Di-n-octyl phthalate is a phthalate ester and a diester.
General Description
A clear liquid with a mild odor. Slightly less dense than water and insoluble in water. Hence floats on water. Flash point 430°F. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. As a liquid, can easily penetrate the soil and contaminate groundwater and nearby streams. Eye contact may produce severe irritation and direct skin contact may produce mild irritation. Used in the manufacture of a variety of plastics and coating products.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
DI-N-OCTYL PHTHALATE reacts with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction with caustic solutions. Flammable hydrogen may be generated by mixing with alkali metals and hydrides. Can generate electrostatic charges by swirling or pouring [Handling Chemicals Safely, 1980. p. 250].
Health Hazard
Produces no ill effects at normal temperatures but may give off irritating vapor at high temperature.
Health Hazard
The acute oral toxicity is very low. Inges tion may result in nausea, somnolence, hallucination, and lacrimation. In humans, sucheffects may be noted at a dose level of150–200 mg/kg. The recovery is prompt.The oral LD50 value in mice is in the range6500 mg/kg. Its irritant action on the skinand the eyes of rabbits was mild. Di-n-octylphthalate fed to mice at the 5% level in dietshowed no reproductive toxicity.
Fire Hazard
Special Hazards of Combustion Products: None
Safety Profile
Mildly toxic by ingestion. Experimental teratogenic and reproductive effects. A skin and severe eye irritant. Used as a plasticizer. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention.Give large quantities of water and induce vomiting. Do notmake an unconscious person vomit.
Source
Detected in distilled water-soluble fractions of new and used motor oil at concentrations of 1.3 to 1.4 and 73 to 78 μg/L, respectively (Chen et al., 1994). May leach from plastic products (e.g., tubing, containers) used in laboratories during chemical analysis of aqueous samples.
Environmental Fate
Biological. o-Phthalic acid was tentatively identified as the major degradation product of di-noctyl
phthalate produced by the bacterium Serratia marcescens (Mathur and Rouatt, 1975). When
di-n-octyl phthalate was statically incubated in the dark at 25 °C with yeast extract and settled
domestic wastewater inoculum, no degradation was observed after 7 d. In a 21-d period, however,
gradual adaptation did occur, resulting in 94 and 93% losses at concentrations of 5 and 10 mg/L,
respectively (Tabak et al., 1981). In the presence of suspended natural populations from
unpolluted aquatic systems, the second-order microbial transformation rate constant determined in
the laboratory was reported to be 3.7 ± 0.6 x 10-13 L/organism?h (Steen, 1991).
Chemical/Physical. Under alkaline conditions, di-n-octyl phthalate will initially hydrolyze to noctyl
hydrogen phthalate and 1-octanol. The monoester will undergo hydrolysis forming ophthalic
acid and 1-octanol (Kollig, 1993). The hydrolysis half-life at pH 7 and 25 °C was
estimated to be 107 yr (Ellington et al., 1988).
storage
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist. Di-noctyl phthalate must be stored to avoid contact with strongoxidizers (such as chlorine, bromine, and fluorine) andstrong acids (such as hydrochloric, sulfuric, and nitric); andalkyl halides, since violent reactions occur. Store in tightlyclosed containers in a cool, well-ventilated area away fromheat. Sources of ignition, such as smoking and open flames,are prohibited where this chemical is used, handled, orstored in a manner that could create a potential fire orexplosion hazard. Metal containers involving the transfer of5 gallons or more of this chemical should be grounded andbonded. Drums must be equipped with self-closing valves,pressure vacuum bungs, and flame arresters. Use only nonsparking tools and equipment, especially when opening andclosing containers of this chemical.
Shipping
The name of this material is not in the DOT listof materials for label and packaging standards. However,based on regulations, it may be classified as anEnvironmentally hazardous substances, liquid, n.o.s. It fallsin Hazard Class 9 and Packing Group III.[20,21]
Properties of DI-N-OCTYL PHTHALATE
| Melting point: | -25℃ |
| Boiling point: | 380 °C |
| Density | 0.980 g/mL at 20 °C(lit.) |
| vapor pressure | 5(x 10-8 mmHg) at 82 °C, 500 at 132 °C (Gross and Colony, 1973) |
| refractive index | n |
| Flash point: | 219 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Oily Liquid |
| color | Colourless |
| Specific Gravity | 0.98 |
| Water Solubility | Insoluble in water. |
| Merck | 14,2864 |
| BRN | 1915994 |
| Henry's Law Constant | 1.41(x 10-12 atm?m3/mol) at 25 °C (approximate - calculated from water solubility and vapor pressure) |
| Dielectric constant | 5.1(24℃) |
| CAS DataBase Reference | 117-84-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Di-n-octyl phthalate(117-84-0) |
| EPA Substance Registry System | Di-n-octyl phthalate (117-84-0) |
Safety information for DI-N-OCTYL PHTHALATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for DI-N-OCTYL PHTHALATE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Di-n-octyl phthalate CAS 117-84-0View Details
Di-n-octyl phthalate CAS 117-84-0View Details
117-84-0 -
 Di-n-octyl phthalate CAS 117-84-0View Details
Di-n-octyl phthalate CAS 117-84-0View Details
117-84-0 -
 Di-n-octyl phthalate CAS 117-84-0View Details
Di-n-octyl phthalate CAS 117-84-0View Details
117-84-0 -
 Di-n-octyl Phthalate CAS 117-84-0View Details
Di-n-octyl Phthalate CAS 117-84-0View Details
117-84-0 -
 Di-n-octyl phthalate CAS 117-84-0View Details
Di-n-octyl phthalate CAS 117-84-0View Details
117-84-0 -
 Di-n-octyl phthalate CAS 117-84-0View Details
Di-n-octyl phthalate CAS 117-84-0View Details
117-84-0 -
 Di-n-octyl phthalate CAS 117-84-0View Details
Di-n-octyl phthalate CAS 117-84-0View Details
117-84-0 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1