Diallyl phthalate
- CAS NO.:131-17-9
- Empirical Formula: C14H14O4
- Molecular Weight: 246.26
- MDL number: MFCD00008646
- EINECS: 205-016-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
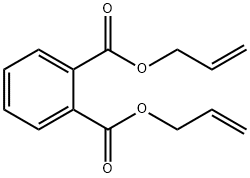
What is Diallyl phthalate?
Chemical properties
clear colourless to light yellow liquid
The Uses of Diallyl phthalate
Diallyl Phthalate is used as a reagent in ring-closing ruthenium based reactions.
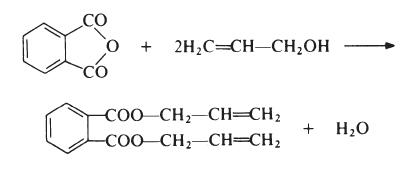
Preparation
Diallyl phthalate (DAP) is prepared by reaction of phthalic anhydride and allyl alcohol:
What are the applications of Application
Diallyl phthalate is an important monomer for the production of thermosetting molding compounds, which must have good dimensional stability and electrical properties, and be resistant to heat and solvents. Diallyl phthalate can be polymerized or copolymerized. This usually is done by dissolving the diallyl phthalate monomer in 2- propanol, adding 50% hydrogen peroxide at about 105 ℃, and precipitating the prepolymer from the cooled, viscous solution with excess 2- propanol. Copolymers containing diallyl phthalate are suitable for specialty coating and for embedding, especially in the production of electronic devices. For example, the moisture-sensitive epoxy compounds now used in light-emitting diode (LED) displays can be replaced by stable diallyl phthalate epoxy encapsulating resins. By adding inorganic materials to diallyl phthalate prepolymer compositions, reinforced thermosetting molding compounds can be obtained. Glass cloth or paper can be impregnated with a solution of prepolymer, monomer, and peroxide initiator. After removal of the solvent, the glass cloth or paper is cured to give the desired film-protected material, which is used for decoration, stain-resistant overlays for household articles, and furniture.
General Description
Clear pale-yellow liquid. Odorless.
Air & Water Reactions
Incompatible with water and oxygen. Should be stored air tight, with inhibitor, to prevent polymerization reaction .
Reactivity Profile
Diallyl phthalate can react with oxidizers. Diallyl phthalate can also react with acids and alkalis. Diallyl phthalate is incompatible with water and oxygen.
Fire Hazard
Diallyl phthalate is combustible.
Flammability and Explosibility
Not classified
Safety Profile
Suspected carcinogen with experimental carcinogenic data. Moderately toxic by ingestion, skin contact, intraperitoneal, and subcutaneous routes. An eye irritant. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidzing materials. To fight fire, use CO2 or dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALLYL COMPOUNDS and ESTERS.
Carcinogenicity
In the 103-week study referred
to previously, a slight increase in MNCL was seen in
female rats treated with 50 or 100 mg/kg/day of DAP. MNCL
occurs in F344 control rats at a high incidence; however, the
incidence of 51% in female rats at the high dose level was
above historical control data for the laboratory (29%). No
significant increases in tumor incidences were seen in male
rats. Based on this study, DAP was considered to have
demonstrated equivocal evidence for carcinogenicity in
female F344 rats according to the NTP.
In male and female B6C3F1 mice receiving 300 mg/kg of
DAP by gavage for 103 weeks (5 days/week), the incidence
of forestomach papillomas was significantly greater than that
of controls. Because of the rarity of forestomach
papillomas in control B6C3F1 mice and the concomitant
observation of dose-related forestomach hyperplasia, the
development of these tumors was considered to be test
substance related. Compared to controls, a slight increase
in the incidence of lymphomas was observed in males
receiving 300 mg/kg/day of DAP. Because the increase
was not statistically significant compared to historical control
data, this effect was not considered to be test substance
related.
Properties of Diallyl phthalate
| Melting point: | -70 °C |
| Boiling point: | 165-167 °C/5 mmHg (lit.) |
| Density | 1.121 g/mL at 25 °C (lit.) |
| vapor density | 8.3 (vs air) |
| vapor pressure | 2.3 mm Hg ( 150 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | 0.18g/l |
| form | Liquid |
| color | Clear colorless to light yellow |
| Odor | mild odor |
| Water Solubility | 6 g/L (20 ºC) |
| BRN | 1880877 |
| CAS DataBase Reference | 131-17-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,2-Benzenedicarboxylic acid, di-2-propenyl ester(131-17-9) |
| EPA Substance Registry System | Diallyl phthalate (131-17-9) |
Safety information for Diallyl phthalate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Diallyl phthalate
| InChIKey | QUDWYFHPNIMBFC-UHFFFAOYSA-N |
Diallyl phthalate manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 DiAllyl Phthalate (DAP) 99%View Details
DiAllyl Phthalate (DAP) 99%View Details -
 Diallyl Phthalate CASView Details
Diallyl Phthalate CASView Details -
 Diallyl Phthalate CASView Details
Diallyl Phthalate CASView Details -
 Diallyl phthalate 95% CAS 131-17-9View Details
Diallyl phthalate 95% CAS 131-17-9View Details
131-17-9 -
 Diallyl phthalate CAS 131-17-9View Details
Diallyl phthalate CAS 131-17-9View Details
131-17-9 -
 Diallyl phthalate 98% (HPLC) CAS 131-17-9View Details
Diallyl phthalate 98% (HPLC) CAS 131-17-9View Details
131-17-9 -
 Liquid Diallyl Phthalate, Packaging Type: BottleView Details
Liquid Diallyl Phthalate, Packaging Type: BottleView Details
131-17-9 -
 Liquid Diallyl Phthalate, For Various, Grade: IndustrialView Details
Liquid Diallyl Phthalate, For Various, Grade: IndustrialView Details
131-17-9
