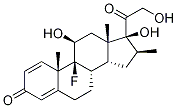
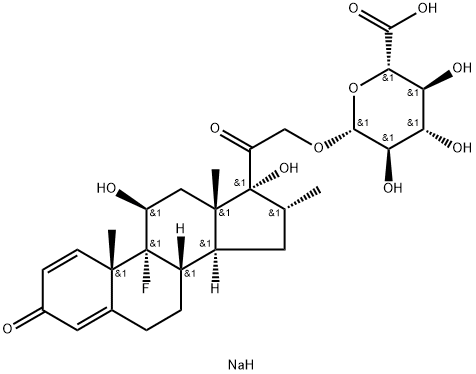
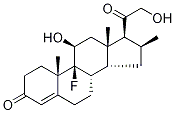
Dexamethasone 21-phosphate disodium salt
Synonym(s):Dexamethasone 21-phosphate disodium salt
- CAS NO.:2392-39-4
- Empirical Formula: C22H28FNa2O8P
- Molecular Weight: 516.4
- MDL number: MFCD00079105
- EINECS: 219-243-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-18 17:17:19
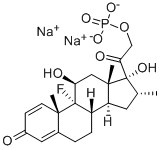
What is Dexamethasone 21-phosphate disodium salt?
Chemical properties
White or almost white, very hygroscopic powder.
Originator
Decadron Phosphate,MS and D,US,1959
The Uses of Dexamethasone 21-phosphate disodium salt
Dexamethasone 21-phosphate disodium salt acts as an inducer of apoptosis and inhibitor of the sodium phosphate symporter. It is used in the treatment of emetic effects of cancer therapy and epicondylitis. Further, it serves as an activator of food, medicine, glucocorticoid and anti inflammatory.
The Uses of Dexamethasone 21-phosphate disodium salt
glucocorticoid, antiinflammatory
The Uses of Dexamethasone 21-phosphate disodium salt
Dexamethasone-21-phosphate disodium salt is used in eye and ear preparations and in systemic preparations.
What are the applications of Application
Dexamethasone Sodium Phosphate is an inducer of apoptosis and inhibitor of the sodium phosphate symporter
Definition
ChEBI: An organic sodium salt which is the disodium salt of dexamethasone phosphate.
Manufacturing Process
A solution of bis-triethylamine phosphate was prepared by slowly adding 2.36
ml of 85% phosphoric acid to 20 ml of acetonitrile containing 9.9 ml of
triethylamine at 20°C. This solution was added to a stirred solution of 4.70 g
of 9α-fluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,2-dione 21-
methanesulfonate and 20 ml of acetonitrile. The mixture was heated under
reflux for four hours and then evaporated under reduced pressure to a volume
of 12 ml. This mixture was a concentrated solution of 9α-fluoro-11β,17α,21-
trihydroxy-16α-methyl-1,4-pregnadiene-3,20-dione 21-phosphate
triethylamine salt with some inorganic phosphate.
The mixture was cooled, 25 ml of methanol added, and the cooled mixture
treated with 33 ml of 1.89 N methanolic sodium methoxide solution. The
precipitated inorganic phosphates were removed by suction filtration and
washed thoroughly with methanol. The combined filtrates were evaporated
under reduced pressure to a volume of 12 ml and treated with 30 ml of
methanol. The resulting cloudy solution was clarified by filtration through
diatomaceous earth. The volume of the filtrate was brought to 40 ml by the
addition of methanol, and 120 ml of ether was added with stirring. The
precipitated product, which was 9α-fluoro-11β,17α,21-trihydroxy-16α-methyl1,4-pregnadiene-3,20-dione 21-phosphate sodium salt, was collected by
suction filtration, and washed with acetone and then with ether. The weight of
the air-dried material was 3.06 g.
brand name
Decadron (Merck); Dexacen (Cent); Dexacort (UCB); Dexair (Pharmafair); Hexadrol (Organon); Maxidex (Alcon).
Therapeutic Function
Glucocorticoid
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion and intraperitoneal routes. Human systemic effects by intravenous route: peritonitis, central nervous system, and gastrointestinal changes. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits toxic fumes of F-, PO,, and Na2O.
Properties of Dexamethasone 21-phosphate disodium salt
| Melting point: | 233-235 °C |
| alpha | D +57° (water); D25 +74 ±4° (calcd on water-free and alcohol-free basis, concn of 10 mg/ml): USP XIX, p 124 |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL, clear, faintly yellow |
| form | White Powder |
| color | white to off-white |
| PH | pH(10g/l, 25℃) : 7.5~10.5 |
| Water Solubility | Soluble in water. Slightly soluble in ethanol. Insoluble in dichloromethane. |
| Merck | 13,2960 |
| BRN | 6473066 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 2392-39-4(CAS DataBase Reference) |
| EPA Substance Registry System | Dexamethasone disodium phosphate (2392-39-4) |
Safety information for Dexamethasone 21-phosphate disodium salt
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Dexamethasone 21-phosphate disodium salt
| InChIKey | PLCQGRYPOISRTQ-FCJDYXGNSA-L |
Dexamethasone 21-phosphate disodium salt manufacturer
Add Biotec
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Dexamethasone disodium phosphate 99%View Details
Dexamethasone disodium phosphate 99%View Details -
 Dexamethasone disodium phosphate 2392-39-4 / 55203-24-2 98%View Details
Dexamethasone disodium phosphate 2392-39-4 / 55203-24-2 98%View Details
2392-39-4 / 55203-24-2 -
 Dexamethasone disodium phosphate 98%View Details
Dexamethasone disodium phosphate 98%View Details
2392-39-4 / 55203-24-2 -
 Dexamethasone disodium phosphate 2392-39-4 / 55203-24-2 98%View Details
Dexamethasone disodium phosphate 2392-39-4 / 55203-24-2 98%View Details
2392-39-4 / 55203-24-2 -
 Dexamethasone sodium phosphate 98% (HPLC) CAS 2392-39-4View Details
Dexamethasone sodium phosphate 98% (HPLC) CAS 2392-39-4View Details
2392-39-4 -
 Dexamethasone sodium phosphate CAS 2392-39-4View Details
Dexamethasone sodium phosphate CAS 2392-39-4View Details
2392-39-4 -
 Dexamethasone sodium phosphate 95.00% CAS 2392-39-4View Details
Dexamethasone sodium phosphate 95.00% CAS 2392-39-4View Details
2392-39-4 -
 Dexamethasone sodium phosphate 99% (HPLC) CAS 2392-39-4View Details
Dexamethasone sodium phosphate 99% (HPLC) CAS 2392-39-4View Details
2392-39-4
