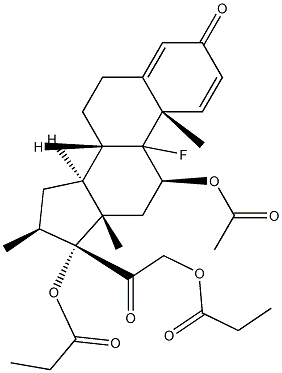
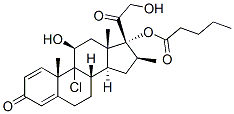
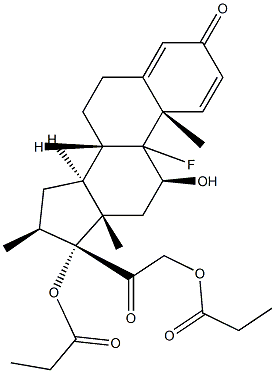
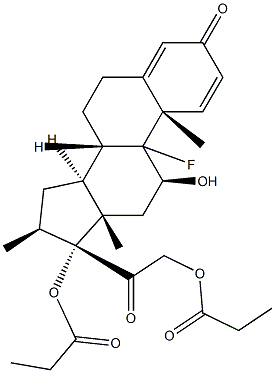
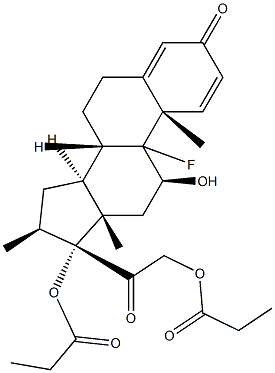
9-Fluoro-11b,17,21-trihydroxy-16a-methylpregna-1,4-diene-3,20-dione 17-valerate
- CAS NO.:33755-46-3
- Empirical Formula: C27H37FO6
- Molecular Weight: 476.58
- MDL number: MFCD00271762
- EINECS: 251-669-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:37
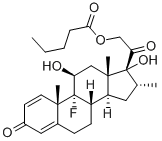
What is 9-Fluoro-11b,17,21-trihydroxy-16a-methylpregna-1,4-diene-3,20-dione 17-valerate?
Originator
Valisone,Schering,US,1967
The Uses of 9-Fluoro-11b,17,21-trihydroxy-16a-methylpregna-1,4-diene-3,20-dione 17-valerate
Dexamethasone Valerate (Betamethasone Valerate EP Impurity C) is an impurity of Dexamethasone (D298800), which is a glucocorticoid that is used as an anti-inflammatory agent. Dexamethasone regulates T cell survival, growth, and differentiation. Dexamethasone inhibits the induction of nitric oxide synthase.
Definition
ChEBI: Dexamethasone valerate is a 21-hydroxy steroid.
Manufacturing Process
The valerate is made from betamethasone as a starting material as follows: A
suspension of 9α-fluoro-11β,17α,21-trihydroxy-16β-methylpregna-1,4-diene-
3,20-dione(betamethasone) (2 grams) in sodium dried benzene (500 ml) was
distilled vigorously for a few minutes, toluene-p-sulfonic acid monohydrate (30
mg) and methyl orthovalerate (5 ml) were added and distillation was
continued for 10 minutes. The mixture was then boiled under reflux for 1.5
hours after which time unreacted betamethasone alcohol (400 mg) was
removed by filtration. The benzene solution was treated with solid sodium
.bicarbonate and a few drops of pyridine, filtered and evaporated to dryness
at about 50°C. The residue, in ether, was filtered through grade III basic
alumina (20grams) to remove traces of unreacted betamethasone alcohol, the
ether removed in vacuo and the residue of crude betamethasone 17,21-
methyl orthovalerate was treated with acetic acid (20ml) and a few drops of
water and left overnight at room temperature.
The acetic acid solution was poured into water (100 ml) and extracted with
chloroform. The chloroform extracts were washed in turn with water, saturated
sodium bicarbonate solution and water, dried and evaporated in vacuo. The
residual gum was triturated with ether and a white crystalline solid (1.16
grams) isolated by filtration. Recrystallization from ether (containing a small
amount of acetone)-petroleum ether gave 9α-fluoro-11β,21-dihydroxy-16β-
methyl-17α-valeryloxypregna-1,4-diene-3,20-dione (871 mg) as fine needles.
Therapeutic Function
Corticosteroid
Properties of 9-Fluoro-11b,17,21-trihydroxy-16a-methylpregna-1,4-diene-3,20-dione 17-valerate
| Melting point: | 150-154°C |
| Density | 1.1174 (estimate) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for 9-Fluoro-11b,17,21-trihydroxy-16a-methylpregna-1,4-diene-3,20-dione 17-valerate
Computed Descriptors for 9-Fluoro-11b,17,21-trihydroxy-16a-methylpregna-1,4-diene-3,20-dione 17-valerate
9-Fluoro-11b,17,21-trihydroxy-16a-methylpregna-1,4-diene-3,20-dione 17-valerate manufacturer
Allmpus Laboratories Pvt Ltd
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran
You may like
-
 33755-46-3 98.62View Details
33755-46-3 98.62View Details
33755-46-3 -
 Avibactam INT 1View Details
Avibactam INT 1View Details
1416134-48-9 -
 AvibactamView Details
AvibactamView Details
1416134-49-0 -
 Avibactam sodium seriesView Details
Avibactam sodium seriesView Details
1192651-49-2 -
 Avibactam seriesView Details
Avibactam seriesView Details
1192651-80-1 -
 Avibactam Sodium SaltView Details
Avibactam Sodium SaltView Details
1192491-61-4 -
 Avibactam intermediateView Details
Avibactam intermediateView Details
1171080-45-7 -
 Relibatan intermediateView Details
Relibatan intermediateView Details
1416134-63-8
