Dexamethasone EP Impurity E
- CAS NO.:13209-41-1
- Empirical Formula: C22H28O4
- Molecular Weight: 356.46
- MDL number: MFCD18452727
- EINECS: 236-177-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-06 13:21:43
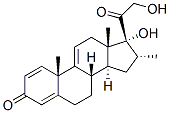
What is Dexamethasone EP Impurity E?
Absorption
Following oral administration with food, the median Tmax of vamorolone is approximately 2 hours.
The co-administration of vamorolone with a high-fat, high-calorie meal reduced Cmax by 18%, increased AUC by 13%, and delayed Tmax by one hour. The co-administration of vamorolone with a low-fat, low-calorie meal reduced Cmax by 4%, increased AUC by 14%, and delayed Tmax by one hour.
Toxicity
Specific data regarding vamorolone overdosage are not readily available. Prescribing information recommends supportive and symptomatic treatment in the event of suspected vamorolone overdose, with consideration given to gastric lavage or emesis if clinically appropriate.
The Uses of Dexamethasone EP Impurity E
A related intermediate of Prednisolone (P703740).
Indications
Vamorolone is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients ≥2 years of age.
Background
Vamorolone is a novel and fully synthetic glucocorticoid developed by Santhera Pharmaceuticals. It is used to manage inflammation and immune dysregulation in patients with Duchenne muscular dystrophy (DMD), a neuromuscular disorder characterized by the insidious regression of neuromuscular function and the most common form of muscular dystrophy in the United States. Corticosteroid therapy is the current standard of care for DMD despite relatively high rates of adverse effects. Vamorolone is positioned as having a more tolerable adverse effect profile than other corticosteroids owing to its unique receptor binding profile, thus providing an additional treatment option in patients for whom corticosteroid adverse effects are intolerable or otherwise unacceptable.
Vamorolone was approved by the FDA in October 2023 for the management of DMD in patients ≥2 years of age.
Pharmacokinetics
In clinical studies, treatment with vamorolone induced a dose-dependent decrease in morning cortisol levels and a dose-dependent increase in leukocyte and lymphocyte counts.
Metabolism
Vamorolone is subject to multiple metabolic pathways, including glucuronidation, hydroxylation, and reduction. Its metabolism is mediated through CYP3A4, CYP3A5, CYP2C8, UGT1A3, UGT2B7, and UGT2B17. Glucuronides - formed via direct glucuronidation and hydrogenation with subsequent glucuronidation - are the main metabolites in the plasma and urine.
Properties of Dexamethasone EP Impurity E
| Melting point: | 228-231 °C(Solv: acetone (67-64-1); hexane (110-54-3)) |
| Boiling point: | 548.3±50.0 °C(Predicted) |
| Density | 1.24±0.1 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Dioxane (Slightly), Ethyl Acetate (Slightly) |
| form | Solid |
| pka | 12.53±0.70(Predicted) |
| color | Pale Yellow |
Safety information for Dexamethasone EP Impurity E
Computed Descriptors for Dexamethasone EP Impurity E
Dexamethasone EP Impurity E manufacturer
Molsyns Research
Vijaya Pharma And Life Science
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran





You may like
-
 13209-41-1 Dexamethasone EP Impurity E 98%View Details
13209-41-1 Dexamethasone EP Impurity E 98%View Details
13209-41-1 -
 Vamorolone 98%View Details
Vamorolone 98%View Details
13209-41-1 -
 13209-41-1 Dexamethasone EP Impurity E 98%View Details
13209-41-1 Dexamethasone EP Impurity E 98%View Details
13209-41-1 -
 Vamorolone CAS 13209-41-1View Details
Vamorolone CAS 13209-41-1View Details
13209-41-1 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0