DESACETYLVINBLASTINEAMIDE
- CAS NO.:53643-48-4
- Empirical Formula: C43H55N5O7
- Molecular Weight: 753.93
- MDL number: MFCD01675265
- EINECS: 258-682-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:39
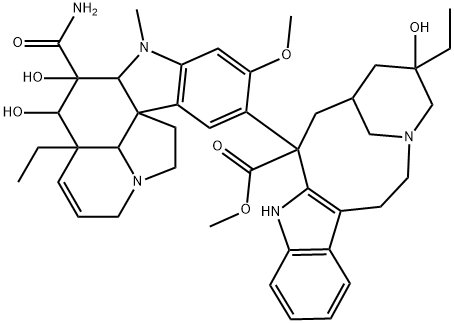
What is DESACETYLVINBLASTINEAMIDE?
Originator
Eldisine,Lilly ,France,1980
The Uses of DESACETYLVINBLASTINEAMIDE
Antineoplastic.
Indications
For the treatment of acute leukaemia, malignant lymphoma, Hodgkin's disease, acute erythraemia and acute panmyelosis
Background
Vinblastine derivative with antineoplastic activity against cancer. Major side effects are myelosuppression and neurotoxicity. Vindesine is used extensively in chemotherapy protocols (antineoplastic combined chemotherapy protocols).
Definition
ChEBI: Vindesine is a vinca alkaloid, a methyl ester, an organic heterotetracyclic compound, an organic heteropentacyclic compound, a tertiary alcohol, a tertiary amino compound and a primary carboxamide. It has a role as an antineoplastic agent. It is functionally related to a vincaleukoblastine.
Manufacturing Process
About 10 g of VLB (vincaleucoblastine or simply vinblastine) sulfate were converted by standard procedures to VLB free base. The free base, obtained as a residue after evaporation of the dried ethereal solvent, was dissolved in about 200 ml of anhydrous methanol. Anhydrous liquid ammonia (300 ml) was added, and the reaction mixture sealed and maintained at about 100°C for 60 hours. The reaction vessel was opened, and the contents removed and evaporated to dryness in vacuo. The resulting residue, containing 4-desacetyI VLB C-3 carboxamide, as shown by thin layer chromatography, were combined and the solvent evaporated therefrom in vacuo, yielding asa residue purified 4-desacetyl VLB C-3 carboxamide free base. The NMR and IR spectra of the solid free base confirmed the structure indicated. The free base showed a band in the infrared at 1,687 cm -l , characteristic of the amide function. The molecular weight of the free base determined by mass spectroscopy was 753 which is in agreement with theoretical value calculated for C 43 H 55 N 5 O 7 .
brand name
Eldisine (Lilly).
Therapeutic Function
Antineoplastic
Pharmacokinetics
Vindesine is indicated for the treatment of acute lymphocytic leukemia of childhood that is resistant to vincristine and non-oat cell lung cancer. Vindesine causes the arrest of cells in metaphase mitosis. It is three times more potent than vincristine and nearly 10 times more potent than vinblastine in causing mitotic arrest in in vitro studies at doses designed to arrest from 10 to 15% of the cells in mitosis. Vindesine and vincristine are approximately equipotent at dose levels that arrest 40 to 50% of the cells in mitosis. Unlike vinblastine, vindesine produces very few postmetaphase cells. Vindesine has demonstrated activity in patients who have relapsed while receiving multiple-agent treatment that included vincristine.
Metabolism
Hepatic
Properties of DESACETYLVINBLASTINEAMIDE
| Melting point: | 230-232° |
| alpha | D25 +39.4° (c = 1.0 in methanol) |
| Density | 1.41±0.1 g/cm3(Predicted) |
| pka | pKa (DMF 66%) 5.39, 7.36; (H2O) 6.04, 7.67(at 25℃) |
Safety information for DESACETYLVINBLASTINEAMIDE
Computed Descriptors for DESACETYLVINBLASTINEAMIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1