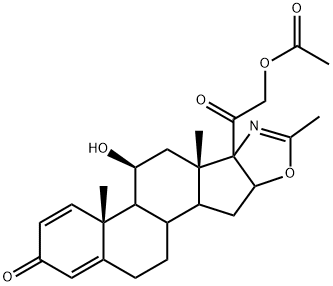
Deflazacort
Synonym(s):(11β,16β)-21-(acetyloxy)-11-hydroxy-2′-methyl-5′H-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione;11β,21-dihydroxy-2′-methyl-5′βH-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione 2′-acetate;azacort;oxazacort;pregna-1,4-diene-11β,21-diol-3,20-dione[17α,16α-d]-2′-methyloxazoline 21-acetate
- CAS NO.:14484-47-0
- Empirical Formula: C25H31NO6
- Molecular Weight: 441.52
- MDL number: MFCD00866106
- EINECS: 238-483-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-26 11:58:42
What is Deflazacort?
Absorption
Deflazacort is rapidly absorbed after oral administration with peak concentration occurring within 1-2 hours. One pharmacokinetic study determined an AUC (area under the curve) of 280 ng/ml · h.
The bioavailability of both the oral suspension and tablet are similar. In clinical studies, coadministration of deflazacort crushed with food or applesauce did not affect absorption or bioavailability.
Toxicity
The LD50 for the oral dose is 5200 mg/kg (mouse); Oral TDLO (woman): 0.12 mg/kg
A note on altered endocrine function and immunosuppression
Deflazacort, as a steroid prodrug used over a long-term period, can cause hormone imbalance leading to diseases such as Cushing's Syndrome and hypothalamic-pituitary-adrenal axis suppression. It can also predispose to infection, as it promotes immunosuppression. It is important to monitor for hormonal imbalance and infection and provide necessary treatment if they occur.
Mutagenicity/carcinogenicity
Mutagenicity assays were negative in various laboratory and in vivo assays performed on rats. Chronic use in mice for 2 years in one study resulted in a higher rate of osteoma and osteosarcomas in mice receiving 0.06, 0.12, 0.25, 0.50, or 1.0 mg/kg of deflazacort daily.
Use in pregnancy
There are no sufficient data to support the administration of deflazacort during pregnancy. Corticosteroid drugs such as deflazacort should only be used during pregnancy only if the benefits of therapy outweigh the potential risks.
Use in lactation
Corticosteroids, when administered systemically, are excreted in the breastmilk. Exposure may lead to disturbances in bone development and growth and endocrine disturbances in the exposed infant.
Description
Deflazacort is an antiinflammatory treatment of rheumatoid arthritis. Its antirheumatic potency i s similar to that of prednisone, while its hyperglycernic and calcium depleting effects are reportedly less.
Chemical properties
Off-White to Pale Yellow Solid
Originator
Dow Lepetit (Italy)
The Uses of Deflazacort
Deflazacort-d3, is the labeled analogue of Deflazacort (D228975), a systemic corticosteroid, and a derivative of prednisolone. Used for rheumatoid arthritis and lupus.
The Uses of Deflazacort
A systemic corticosteroid. A derivative of prednisolone. Used for rheumatoid arthritis and lupus
The Uses of Deflazacort
antiinflammatory
Indications
Deflazacort is indicated for the treatment of Duchenne Muscular Dystrophy (DMD) in patients 2 years of age and older.
What are the applications of Application
Deflazacort is a systemic corticosteroid
Background
Deflazacort, also known as Emflaza, is a corticosteroid prodrug used as an agent to manage Duchenne Muscular Dystrophy (DMD). It is marketed by Marathon Pharmaceuticals and was approved in February 2017 by the FDA.
Duchenne Muscular Dystrophy is an inherited disorder resulting from mutations of the dystrophin gene, which is important for muscle function. This disease can cause serious muscle weakness and progressive breathing and cardiovascular disability, severely impacting patient quality of life and survival. This disease usually manifests by muscle weakness in early childhood followed by loss of the ability to walk (ambulation) as early as age 7.
Deflazacort delays the onset of muscle related complications resulting from DMD, prolonging the lives of children diagnosed with this disease and exerting less harmful effects on the bone health and weight than other steroid medications.
Definition
ChEBI: Deflazacort is a corticosteroid hormone.
brand name
LANTADIN; DEFLAN
Biochem/physiol Actions
Deflazacort is an anti-inflammatory and immunosuppressant glucocorticoid.
Pharmacokinetics
Deflazacort exerts anti-inflammatory activity in DMD, likely improving various symptoms, including muscle weakness and cardiorespiratory symptoms in addition to delaying their onset. This allows for an increased quality of life and prevents the necessity for surgical procedures, such as those for scoliosis, which is associated with DMD. Studies showed significant preservation of muscle mass in patients generally treated with 0.9 mg/kg/day of deflazacort compared to a control group. The following findings are based on clinical studies using deflazacort on a long term basis:
Effects on muscle strength
At age 16, individuals treated with long-term deflazacort had 63 ± 4% score in muscle strength compared to a mean muscle strength score of 31 ± 3% for control patients. Significant improvements in climbing stairs and rising from a supine position were also seen in patients taking deflazacort.
Effects on ambulation
Ambulation was significantly higher by 12 years of age and 18 years of age in patients taking deflazacort when compared with the control group. The control group showed a mean loss of ambulation of 2 years sooner than with deflazacort treatment.
Effects on cardiac function
Mean left ventricular ejection fraction (a measure of cardiac function) was higher in patients treated with deflazacort over the long term. Preservation of cardiac function was demonstrated by a mean difference in ejection fraction of about 7%, favoring study groups taking deflazacort over control groups.
Effects on spinal alignment
Children treated with deflazacort also significantly lowered the rate and severity of scoliosis and eliminated the need for scoliosis surgery after long-term treatment.
Clinical Use
Glucocorticoid:
Suppression of inflammatory and allergic disorders
Drug interactions
Potentially hazardous interactions with other drugs
Aldesleukin: avoid concomitant use
Antibacterials: metabolism accelerated by rifamycins;
metabolism possibly inhibited by erythromycin;
concentration of isoniazid possibly reduced.
Anticoagulants: efficacy of coumarins and
phenindione may be altered.
Antiepileptics: metabolism accelerated by
carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone
Antifungals: increased risk of hypokalaemia with
amphotericin - avoid; metabolism possibly inhibited
by itraconazole and ketoconazole
Antivirals: concentration possibly increased by
ritonavir.
Ciclosporin: rare reports of convulsions in patients
on ciclosporin and high-dose corticosteroids;
increased half-life of deflazacort.
Cobicistat: concentration increased by cobicistat -
avoid.
Diuretics: enhanced hypokalaemic effects of
acetazolamide, loop diuretics and thiazide diuretics.
Vaccines: high dose corticosteroids can impair
immune response to vaccines; avoid with live
vaccines.
Metabolism
After oral ingestion, deflazacort is deacetylated at position 21 by plasma esterases, producing the active metabolite 21-deflazacort. 21-deflazacort is then further metabolized by CYP3A4 to inactive metabolite products. Deflazacort 21-OH metabolism is extensive. The metabolite of deflazacort-21-OH is deflazacort 6-beta-OH.
Metabolism
Deflazacort is immediately converted by plasma esterases to the pharmacologically active metabolite (D 21-OH). It is 40% protein-bound and has no affinity for corticosteroid-binding-globulin (transcortin). Elimination takes place primarily through the kidneys; 70% of the administered dose is excreted in the urine. The remaining 30% is eliminated in the faeces. Metabolism of D 21-OH is extensive; only 18% of urinary excretion represents D 21-OH. The metabolite of D 21-OH, deflazacort 6-beta-OH, represents one third of the urinary elimination.
Properties of Deflazacort
| Melting point: | 255-256.5°C |
| Boiling point: | 595.4±50.0 °C(Predicted) |
| alpha | D +62.3° (c = 0.5 in chloroform) |
| Density | 1.41 |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥20mg/mL |
| pka | 14.30±0.70(Predicted) |
| form | powder |
| color | white to tan |
| Merck | 14,2862 |
Safety information for Deflazacort
Computed Descriptors for Deflazacort
| InChIKey | FBHSPRKOSMHSIF-UBRVQRTASA-N |
| SMILES | C12(C(COC(C)=O)=O)[C@]3(C)C(C4C([C@H](C3)O)[C@]3(C)C(=CC(C=C3)=O)CC4)CC1OC(C)=N2 |
Deflazacort manufacturer
Dayaram Pharma Chem
Stermone Chemicals Pvt Ltd
Maharshi Pharma Chem
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
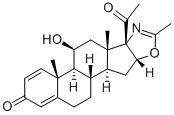
You may like
-
 Deflazacort 98%View Details
Deflazacort 98%View Details -
 Deflazacort 99%View Details
Deflazacort 99%View Details -
 Deflazacort 98%View Details
Deflazacort 98%View Details
14484-47-0 -
 Deflazacort CAS 14484-47-0View Details
Deflazacort CAS 14484-47-0View Details
14484-47-0 -
 Deflazacort 14484-47-0 99%View Details
Deflazacort 14484-47-0 99%View Details
14484-47-0 -
 Deflazacort 99%View Details
Deflazacort 99%View Details -
 Deflazacort 98.00% CAS 14484-47-0View Details
Deflazacort 98.00% CAS 14484-47-0View Details
14484-47-0 -
 Deflazacort CAS 14484-47-0View Details
Deflazacort CAS 14484-47-0View Details
14484-47-0