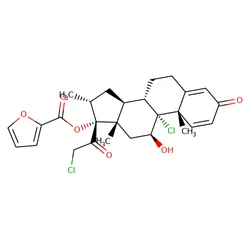
Mometasone furoate
Synonym(s):(11β,16α)-9,21-Dichloro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-16-methylpregna-1,4-diene-3,20-dione;9α,21-dichloro-11β-hydroxy-16α-methyl 3,20-dioxopregna-1,4-dien-17-yl furan-2-carboxylate;Mometasone furoate
- CAS NO.:83919-23-7
- Empirical Formula: C27H30Cl2O6
- Molecular Weight: 521.43
- MDL number: MFCD00866003
- EINECS: 617-501-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
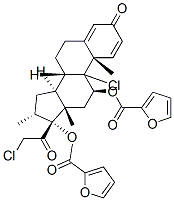
What is Mometasone furoate?
Absorption
The mean time to peak concentration is 1.0 to 2.5 hours. Bioavailability has been reported as <1% but studies of repeat doses of inhaled corticosteroids suggest a bioavailability of 11%. The 0.1% ointment may have a bioavailability of 0.7%.
Toxicity
Overdose with a mometasone furoate inhaler may occur with chronic overuse. Symptoms of chronic overuse may present as hypercorticism and adrenal suppression, and patients may not require any more treatment than monitoring.
In animal studies of pregnancy, some fetal toxic effects were seen at or above the maximum recommended human dose, though rodents are more sensitive to these effects than humans. The benefits and risks of use should be considered in pregnant patients
It is unknown if mometasone furoate is excreted in breast milk but other corticosteroids are and therefore caution should be exercised when administering to nursing mothers.
Safety and effectiveness in pediatric populations has been established through clinical trials, though there may be a reduction in expected growth of about 1cm per year depending on the dose and duration of treatment. Pediatric patients should be titrated to the lowest effective dose for mometasone furoate inhalers.
A trial of geriatric patients showed no difference in safety or efficacy compared to younger patients, however patients of an even greater age may still be more sensitive to mometasone furoate.
The use of a mometasone furoate inhaler in moderate or severe hepatic impairment rarely leads to detectable plasma concentrations though caution may be prudent with increasing degrees of severity.
The effects of mometasone furoate in renal impairment, and across gender and race have not been studied.
Description
Mometasone furoate is a topical steroidal antiinflammatory useful in the treatment of corticosteroid-responsive dermatoses. It is a highpotency GC available in cream, lotion, or ointment formulationsfor topical use. In addition, mometasone furoatemonohydrate is formulated for treating allergic rhinitis andasthma. Mometasone furoate is formulated as a dry powder inhaler, nasal spray, and ointment for its different indications.
Indications
Inhaled mometasone furoate is indicated for prophylaxis of asthma in patients ≥4 years. Applied topically as an ointment, mometasone furoate is indicated for symptomatic treatment of dermatitis and pruritis in patients ≥2 years.
Mometasone furoate nasal spray is available both over-the-counter (OTC) and by prescription. The OTC nasal spray formulation of mometasone furoate is indicated for the treatment of upper respiratory allergic symptoms (e.g. rhinorrhea, sneezing) in patients ≥2 years of age. The prescription formulation is indicated for the treatment of chronic rhinosinusitis with nasal polyps in patients ≥18 year old and for the and prophylaxis of seasonal allergic rhinitis in patients ≥12 years old. It is also approved in combination with olopatadine for the symptomatic treatment of seasonal allergic rhinitis in patients ≥12 years.
Definition
ChEBI: Mometasone furoate is a 2-furoate ester, a steroid ester, an 11beta-hydroxy steroid, a 20-oxo steroid, an organochlorine compound and a 3-oxo-Delta(1),Delta(4)-steroid. It has a role as an anti-inflammatory drug and an anti-allergic agent. It is functionally related to a mometasone.
Chemical properties
Mometasone furoate is a white powder with a molecular weight of 521.44 kD. It is a potent glucocor ticoid, with a higher binding affinity to the glucocorticoid receptor that is 1.5 times that of fluticasone, 5 times that of budesonide, 7 times that of triamcinolone acetonide, and 12 times that of dexamethasone.
Biological Functions
Mometasone furoate is a 17-heterocyclic intranasal corticosteroid. It has strong local anti-inflammatory activity equivalent to that of fluticasone propionate. Mometasone furoate is also used as an adjunct to anti-bacterials for treating acute rhinosinusitis. In addition, it relieves the symptoms of asthma in both adults and adolescents by exhibiting a broad spectrum of anti-inflammatory properties.
Pharmacokinetics
Mometasone is a synthetic corticosteroid with an affinity for glucocorticoid receptors 22 times higher than that of dexamethasone. Mometasone furoate also has a lower affinity to mineralocorticoid receptors than natural corticosteroids, making it more selective in its action. Mometasone furoate diffuses across cell membranes to activate pathways responsible for reducing inflammation.
Clinical Use
Mometasone furoate dry powder inhaler (MF-DPI) is avail able in more than 40 countries for the treatment of asthma; it was approved in the USA in 2005 for patients 12 years and older, and in 2008 it was approved for patients aged 4-- 11 years. It comes in two strengths, 110 and 220 μg. It is dispensed via a Twisthaler and delivers a consistent amount of product at a variety of flow rates, which takes into account varied asthma severity which can affect overall inspiratory flow and peak inspiratory flow. In vitro testing found that MF-DPI doses were delivered consistently at a flow rate as low as 28.3 liters/min.
Mechanism of action
Mometasone furoate and other cortico steroids relieve the symptoms of asthma through a broad spectrum of anti-inflammatory effects. They re ducethenumberofinflammatorycellsinthelungs, inhibit the synthesis and release of inflammatory mediators,reducebronchialhyper-responsiveness, minimise mucus secretion and may prevent or re verse β2-adrenoceptor down-regulation associated with long term β2-adrenoceptor agonist usage.
Metabolism
Metabolism of mometasone furoate is largely performed hepatically by cytochrome P450 3A4 producing a number of metabolites. Some of these metabolites include free mometasone and 6-beta-hydroxy-mometasone furoate.
Properties of Mometasone furoate
| Melting point: | 218-220°C |
| Boiling point: | 655.5±55.0 °C(Predicted) |
| alpha | D26 +58.3° (dioxane) |
| Density | 1.37±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥20mg/mL |
| pka | 13.02±0.70(Predicted) |
| form | powder |
| color | white to off-white |
| optical activity | [α]/D +50 to +60°, c = 0.5 in methanol |
| CAS DataBase Reference | 83919-23-7(CAS DataBase Reference) |
Safety information for Mometasone furoate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. P362:Take off contaminated clothing and wash before reuse. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Mometasone furoate
| InChIKey | WOFMFGQZHJDGCX-ZULDAHANSA-N |
| SMILES | C[C@]12C[C@H](OC(=O)C3=CC=CO3)C3([C@](CCC4[C@]3(C)C=CC(=O)C=4)([H])[C@]1([H])C[C@@H](C)[C@]2(OC(=O)C1OC=CC=1)C(=O)CCl)Cl |&1:1,3,13,17,25,28,30,r| |
Mometasone furoate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

![(16α)-21-Chloro-17-[(2-furanylcarbonyl)oxy]-16-Methyl-pregna-1,4-diene-3,11,20-trione](https://img.chemicalbook.in/CAS/GIF/1305334-31-9.gif)


You may like
-
 Mometasone Furoate 98%View Details
Mometasone Furoate 98%View Details -
 Mometasone furoate 99%View Details
Mometasone furoate 99%View Details -
 83919-23-7 98%View Details
83919-23-7 98%View Details
83919-23-7 -
 Mometasone Furoate 99%View Details
Mometasone Furoate 99%View Details -
 MOMETASONE FUROATE API, BPView Details
MOMETASONE FUROATE API, BPView Details
83919-23-7 -
 CAS 83919 23 7 MOMETASONE FUROATE, 15 gmView Details
CAS 83919 23 7 MOMETASONE FUROATE, 15 gmView Details
83919-23-7 -
 Mometasone Furoate Powder, USPView Details
Mometasone Furoate Powder, USPView Details
83919-23-7 -
 Mometasone Furoate Api, USPView Details
Mometasone Furoate Api, USPView Details
83919-23-7
