Decane
Synonym(s):Decane;extraction;n-Decane
- CAS NO.:124-18-5
- Empirical Formula: C10H22
- Molecular Weight: 142.28
- MDL number: MFCD00008954
- EINECS: 204-686-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:53
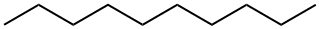
What is Decane?
Chemical properties
colourless liquid
Chemical properties
Decane, C10H22, is a flammable liquid with specific gravity 0.73. Decane is a constituent in the paraffin fraction of crude oil and natural gas. It is released to the environment via the manufacture, use, and disposal of many products associated with the petroleum, gasoline, and plastics industries.
Physical properties
Clear, colorless liquid. Reported odor threshold concentrations were 11.3 mg/m3 by Laffort and Dravnieks (1973) and 620 ppbv by Nagata and Takeuchi (1990).
The Uses of Decane
Decane is obtained mainly from the refining of petroleum. It is a component of engine fuel and is used in organic synthesis, as a solvent, as a standardized hydrocarbon, and in jet fuel research.
The Uses of Decane
Internal standard in the GC analysis of oxalic, malonic, and succinic acids in biological materials.
The Uses of Decane
Decane is a constituent in the paraffin fraction of petroleum and is also present in low concentrations as a component of gasoline. It is used as a solvent in organic synthesis reactions as a hydrocarbon standard in the manufacture of petroleum products, in the rubber industry, and in the paper processing industry, and as a constituent in polyolefin manufacturing wastes. Decane is a flammable liquid (at room temperature) that is lighter than water.
Definition
ChEBI: Decane is a straight-chain alkane with 10 carbon atoms.
Reactions
The Combustion Reaction of Decane is as follows:
Like all hydrocarbons, decane undergoes hydrocarbon combustion when used as a fuel. The balanced chemical equation for the complete combustion of decane is:
2 C10H22 + 31 O2 → 20 CO2 + 22 H2O + Heat Energy (Enthalpy)
The hydrocarbon combustion reaction releases heat energy and is an example of an exothermic reaction. The reaction also has a negative enthalpy change (ΔH) value.
Synthesis Reference(s)
The Journal of Organic Chemistry, 43, p. 2259, 1978 DOI: 10.1021/jo00405a036
Tetrahedron Letters, 34, p. 3745, 1993 DOI: 10.1016/S0040-4039(00)79216-1
General Description
A colorless liquid. Flash point 115°F. Less dense than water and insoluble in water. Vapors heavier than air. In high concentrations its vapors may be narcotic. Used as a solvent and to make other chemicals.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
DECANE is incompatible with oxidizing agents.
Health Hazard
Contact with eyes may produce mild irritation. Contact with skin may cause defatting, redness, scaling, and hair loss. Ingestion may cause diarrhea, slight central nervous system depression, difficulty in breathing and fatigue. Inhalation of high concentrations may cause rapid breathing, fatigue, headache, dizziness, and other CNS effects.
Fire Hazard
Special Hazards of Combustion Products: May produce toxic fumes, including carbon monoxide.
Flammability and Explosibility
Flammable
Biochem/physiol Actions
Decane-1,2-diol derivative might prevent cancer development.
Safety Profile
Questionable carcinogen with experimental tumorigenic data. A simple asphyxiant. Narcotic in high concentrations, Flammable liquid when exposed to heat or flame. Can react with oxidizing materials. Moderately explosive in its vapor form. To fight fire, use foam, CO2, dry chemical. Emitted from modern buildtng materials (CENEAR 69,22,91). See also ARGON for discussion of asphyxiants.
Carcinogenicity
Mice treated with decane developed tumors on the backs, after exposure to ultraviolet radiation at wavelengths longer than 350 nm, generally considered noncarcinogenic. A series of 21 tobacco smoke components and related compounds were applied to mouse skin (50 female ICR/Ha Swiss mice/group) three times weekly with 5 mug/application of benzo[a]pyrene (B[a]P). The test compounds were of five classes: aliphatic hydrocarbons, aromatic hydrocarbons, phenols, and longchain acids and alcohols. Decane was among the compounds that enhanced remarkably the carcinogenicity of B[a]P. and also acted as tumor promoter in two-stage carcinogenesis.
Source
Major constituent in paraffin (quoted, Verschueren, 1983).
Identified as one of 140 volatile constituents in used soybean oils collected from a processing
plant that fried various beef, chicken, and veal products (Takeoka et al., 1996).
California Phase II reformulated gasoline contained decane at a concentration of 1,120 mg/kg.
Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without catalytic
converters were 300 and 42,600 μg/km, respectively (Schauer et al., 2002).
Environmental Fate
Biological. Decane may biodegrade in two ways. The first is the formation of decyl
hydroperoxide, which decomposes to 1-decanol, followed by oxidation to decanoic acid. The other
pathway involves dehydrogenation to 1-decene, which may react with water giving 1-decanol
(Dugan, 1972). Microorganisms can oxidize alkanes under aerobic conditions (Singer and
Finnerty, 1984). The most common degradative pathway involves the oxidation of the terminal
methyl group forming the corresponding alcohol (1-decanol). The alcohol may undergo a series of
dehydrogenation steps, forming decanal, followed by oxidation forming decanoic acid. The fatty
acid may then be metabolized by β-oxidation to form the mineralization products, carbon dioxide
and water (Singer and Finnerty, 1984). Hou (1982) reported 1-decanol and 1,10-decanediol as
degradation products by the microorganism Corynebacterium.
Photolytic. A photooxidation reaction rate constant of 1.16 x 10-11 cm3/molecule?sec was
reported for the reaction of decane with OH in the atmosphere (Atkinson, 1990).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water vapor. Decane
will not hydrolyze because it has no hydrolyzable functional group.
Purification Methods
It can be purified by shaking with conc H2SO4, washing with water, aqueous NaHCO3, and more water, then drying with MgSO4, refluxing with Na and distilling. Also purify through a column of silica gel or alumina. It has been purified by azeotropic distillation with 2-butoxyethanol, the alcohol being washed out of the distillate, using water, the decane is next dried and redistilled. It can be stored with NaH. Further purification can be achieved by preparative gas chromatography on a column packed with 30% SE-30 (General Electric methyl-silicone rubber) on 42/60 Chromosorb P at 150o and 40psig, using helium [Chu J Chem Phys 41 226 1964]. It is soluble in EtOH and Et2O. [Beilstein 1 IV 484.]
Toxicity evaluation
If released to air, n-decane will exist solely as a vapor in the
ambient atmosphere. Vapor-phase n-decane will be degraded
in the atmosphere by reaction with photochemically produced
hydroxyl radicals, the half-life for this reaction in air is
approximately 11.5 h.
Based on n-decane’s vapor pressure, it may volatilize from
dry soil surfaces. In biodegradation studies in soil, hydrocarbons
with molecular weights equivalent to or lower than
decane disappeared from the soil in both active and sterile
treatments by the first sampling time (5 days), indicating that
evaporation was a major removal process. Decane is expected
to have low to no mobility in soil based on estimated organic
carbon partition coefficient (Koc) values in the range of 1700–
43 000. Volatilization from moist soil surfaces is expected to be
an important fate process based on a Henry’s law constant of
5.2 atm m3 mol-1, which is calculated from n-decane’s vapor
pressure and water solubility. Adsorption to soil, however,
would be expected to attenuate due to volatilization.
Properties of Decane
| Melting point: | -30 °C |
| Boiling point: | 174 °C(lit.) |
| Density | 0.735 |
| vapor density | 4.9 (vs air) |
| vapor pressure | 1 mm Hg ( 16.5 °C) |
| refractive index | n |
| Flash point: | 115 °F |
| storage temp. | Store below +30°C. |
| solubility | 0.00005g/l |
| form | Liquid |
| color | Colorless |
| Specific Gravity | 0.731 (20/4℃) |
| Relative polarity | -0.3 |
| Odor Threshold | 0.87ppm |
| explosive limit | 0.7-5.4%(V) |
| Water Solubility | INSOLUBLE |
| BRN | 1696981 |
| Henry's Law Constant | 5.59 at 25 °C (calculated from water solubility and vapor pressure, Tolls, 2002) |
| Dielectric constant | 1.8(130℃) |
| Stability: | Stable. Incompatible with oxidizing agents. Flammable. |
| CAS DataBase Reference | 124-18-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Decane(124-18-5) |
| EPA Substance Registry System | Decane (124-18-5) |
Safety information for Decane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H304:Aspiration hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Decane
| InChIKey | DIOQZVSQGTUSAI-UHFFFAOYSA-N |
Decane manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 n-Decane CAS 124-18-5View Details
n-Decane CAS 124-18-5View Details
124-18-5 -
 n-Decane CAS 124-18-5View Details
n-Decane CAS 124-18-5View Details
124-18-5 -
 n-Decane CAS 124-18-5View Details
n-Decane CAS 124-18-5View Details
124-18-5 -
![Decane [Standard Material for GC] CAS 124-18-5](https://img.chemicalbook.in//Content/image/CP5.jpg) Decane [Standard Material for GC] CAS 124-18-5View Details
Decane [Standard Material for GC] CAS 124-18-5View Details
124-18-5 -
 Decane 95% CAS 124-18-5View Details
Decane 95% CAS 124-18-5View Details
124-18-5 -
 Decane CAS 124-18-5View Details
Decane CAS 124-18-5View Details
124-18-5 -
 Decane, 98% CAS 124-18-5View Details
Decane, 98% CAS 124-18-5View Details
124-18-5 -
 Decane, puriss CAS 124-18-5View Details
Decane, puriss CAS 124-18-5View Details
124-18-5