Darifenacin hydrobromide
Synonym(s):(3S)-1-[2-(2,3-Dihydro-5-benzofuranyl)ethyl]-α,α-diphenyl-3-pyrrolidineacetamide hydrobromide;UK 88525-04
- CAS NO.:133099-07-7
- Empirical Formula: C28H31BrN2O2
- Molecular Weight: 507.47
- MDL number: MFCD08141803
- EINECS: 603-705-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
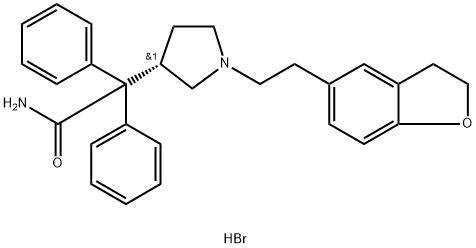
What is Darifenacin hydrobromide?
Description
Darifenacin, an orally active, once a day selective M3 receptor antagonist, was launched for the treatment of overactive bladder in patients with symptoms of urge urinary incontinence, urgency and frequency. The drug selectively inhibits M3 receptor in the detrusor muscle while sparing the M1 and M2 receptors that are believed to be involved in central nervous system and cardiovascular function respectively. The compound was originally developed by Pfizer and licensed to Novartis and Bayer.
Description
Darifenacin is an antagonist of M3 muscarinic acetylcholine receptors (mAChRs; Ki = 0.76 nM). It is selective for M3 over M1, M2, M4, and M5 mAChRs (Kis = 7.08, 44.67, 45.71, and 9.33 nM, respectively). Darifenacin selectively inhibits contractions in isolated guinea pig ileum, bladder, and trachea (pA2s = 9.44, 8.66, and 8.7, respectively), tissues that endogenously express high levels of M3 mAChRs, over isolated rabbit vas deferens and isolated guinea pig atria (pA2s = 7.9 and 7.48, respectively), which endogenously express M1 and M2 mAChRs, respectively. It inhibits micturition pressure (ED50 = 0.089 mg/kg, i.v.), as well as micturition interval and volume in rats. Formulations containing darifenacin have been used in the treatment of overactive bladder.
Chemical properties
White Solid
The Uses of Darifenacin hydrobromide
Darifenacin hydrobromide is used as a medication to treat urinary incontinence. It works by blocking the M3 muscarinic acetylcholine receptor.
The Uses of Darifenacin hydrobromide
calcium replenisher
The Uses of Darifenacin hydrobromide
acute lymphoblastic leukemia therapeutic
What are the applications of Application
Darifenacin Hydrobromide is an M3 muscarinic acetylcholine receptor blocker
Definition
ChEBI: The hydrobromide salt of darifenacin. A selective antagonist for the M3 muscarinic acetylcholine receptor, which is primarily responsible for bladder muscle contractions, it is used in the management of urinary incontinence.
brand name
Enablex (Novartis).
General Description
Darfenacin (Enablex),(s)-2-{1-[2-(2,3-dihydrobenzofuran-5-yl)ethyl]-3-pyrrolidinyl}-2,2-diphen-ylacetamide, is an antimuscarinicagent that has selectivity for the M3 muscarinicsubtype receptor. By competitively blocking of the muscarinicreceptors results in a reduction of the smoothmuscle tone, allowing for greater volume of urine to bestored in the bladder. This results in less urinary incontinence,urgency, and frequency. It is a white to almostwhite, crystalline powder, with a molecular weight of507.5. Darifenacin is metabolized by the isozymesCYP2D6 and CYP3A4 with the primary metabolic routesbeing monohydroxylation of the dihydrobenzofuran ring,opening of the dihydrobenzofuran ring, and N-dealkylationof the pyrrolidine nitrogen.
Biochem/physiol Actions
Darifenacin hydrobromide is an antispasmodic muscarinic antagonist, selective for blocking the M3 muscarinic acetylcholine receptor, which is primarily responsible for bladder muscle contractions. Darifenacin hydrobromide has 9 and 12-fold greater affinity for M3 compared to M1 and M5, respectively, and 59-fold greater affinity for M3 compared to both M2 and M4. Darifenacin is used clinically to treat urinary incontinence and overactive bladder syndrome.
Synthesis
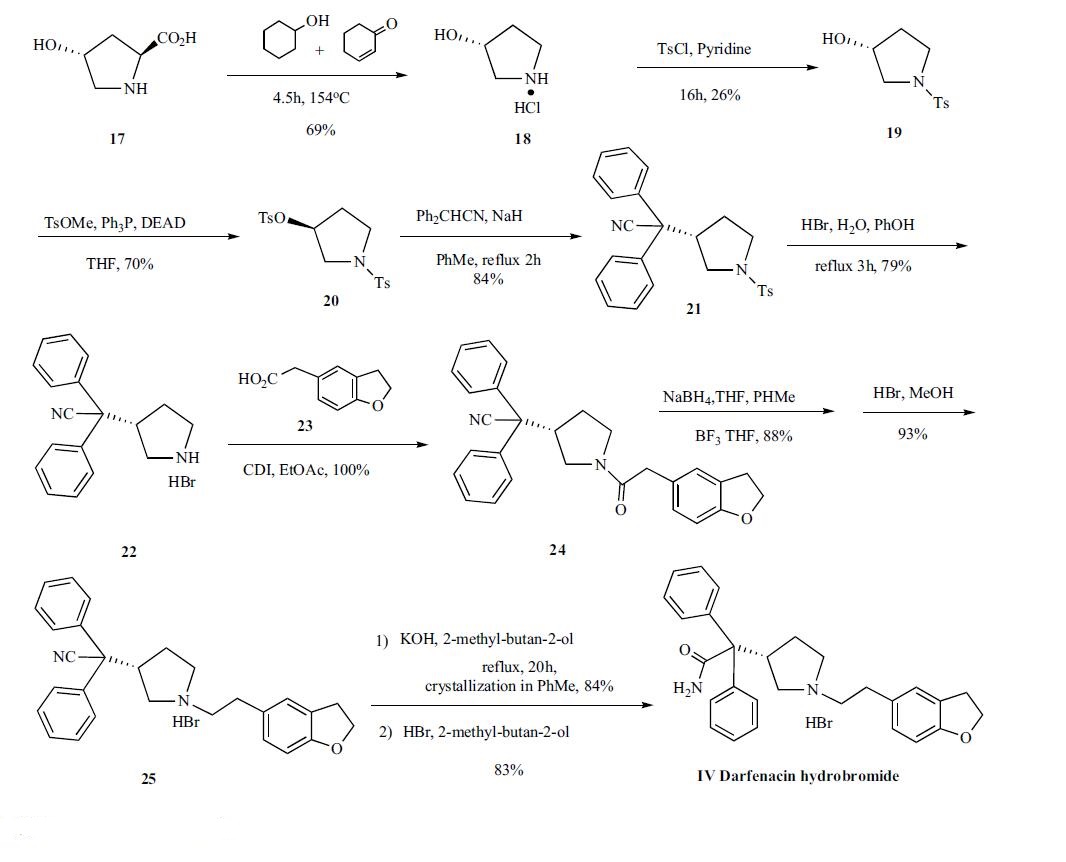
The synthesis of darifenacin is depicted in Scheme 5. Commercially available (2S,4R)-(-)-4-hydroxy-2-pyrrolidinecarboxylic acid (17), anhydrous cyclohexanol and 2-cyclohexen-1-one were heated at 154oC to give de-carboxylated compound 18 in 69 % yield. The 3-(R)-hydroxypyrrolidine (18) was N-tosylated with p-toluenesulfonyl chloride in pyridine yielding compound 19 in 26 % yield . The N-tosylated alcohol 19 was subjected to Mitsunobu reaction in the presence of methyl ptoluenesulfonate, triphenylphosphine and diethyl azodicarboxylate (DEAD) in THF to afford N-tosyl-3(S)-(tosyloxy) pyrrolidine (20) in 70% yield, which was then condensed with 2,2-diphenylacetonitrile with NaH in refluxing toluene to give 2,2-diphenyl-2-[1-(p-toluenesulfonyloxy)pyrrolidin- 2(S)-yl]acetonitrile (21). The tosyl group of 21 was removed with 48% HBr and phenol in refluxing water to yield 2,2- diphenyl-2-[2(S)-pyrrolidinyl] acetonitrile as its corresponding hydrogen bromide salt (22), which was coupled to 2-(2, 3-dihydrobenzofuran-5-yl) acetic acid (23) by treatment with carbonyldiimidazole (CDI) in ethyl acetate to the corresponding amide 24 in a quantitative yield. The amide (24) was dissolved in toluene and reduced with sodium borohydride in THF with slow addition of boron trifluoride THF complex to keep the temperature below 10??C to give free amine in 88% yield. The free amine was converted to corresponding hydrogen bromide salt (25) with 48% HBr in methanol. Compound 25 was hydrolyzed with potassium hydroxide in refluxing 2-methyl-butan-2-ol for twenty hours to give acetamide which was crystallized from toluene as a toluene solvated form in 84% yield. Finally, the toluene solvated compound was converted to darfenacin hydrobromide (IV) with 48% HBr in 2-methyl-butan-2-ol.
References
[1]. hegde ss1,choppin a,bonhaus d,briaud s,loeb m,moy tm,loury d,eglen rm.functional role of m2 andm3muscarinic receptorsin the urinary bladder of rats in vitro and in vivo.br j pharmacol.1997 apr;120(8):1409-18.
[2]. brann mr1,ellis j,jrgensen h,hill-eubanks d,jones sv. muscarinicacetylcholinereceptorsubtypes: localization and structure/function.prog brain res.1993;98:121-7.
[3]. miller dw1,hinton m,chen f.evaluation of drug efflux transporter liabilities of darifenacin in cell culture models of the blood-brain and blood-ocular barriers. neurourol urodyn.2011 nov;30(8):1633-8. doi: 10.1002/nau.21110. epub 2011 aug 8.
[4]. haab f1,stewart l,dwyer p.darifenacin, anm3selectivereceptorantagonist, is an effective and well-tolerated once-daily treatment for overactive bladder. eur urol.2004 apr;45(4):420-9; discussion 429.
Properties of Darifenacin hydrobromide
| Melting point: | 228-2300C |
| alpha | 25D -30.3° (c = 1.0 in methylene chloride) |
| storage temp. | -20°C |
| solubility | DMSO: soluble20mg/mL, clear |
| form | powder |
| color | white to beige |
| optical activity | [α]/D +41 to +49°, c = 1 in methylene chloride |
| CAS DataBase Reference | 133099-07-7(CAS DataBase Reference) |
Safety information for Darifenacin hydrobromide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Darifenacin hydrobromide
Darifenacin hydrobromide manufacturer
Apotex Pharmachem India Pvt Ltd
Macleods Pharmaceuticals Limited
HRV Global Life Sciences
Megafine Pharma
Ralington Pharma
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

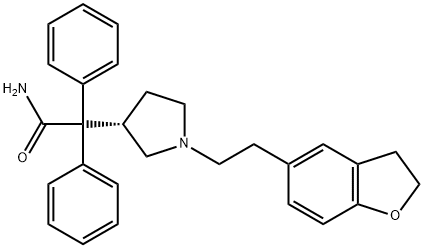


You may like
-
 133099-07-7 98%View Details
133099-07-7 98%View Details
133099-07-7 -
 Darifenacin hydrobromide 98%View Details
Darifenacin hydrobromide 98%View Details
133099-07-7 -
 Darifenacin hydrobromide 97% CAS 133099-07-7View Details
Darifenacin hydrobromide 97% CAS 133099-07-7View Details
133099-07-7 -
 133099-07-7 Darifenacin hydrobromide 98%View Details
133099-07-7 Darifenacin hydrobromide 98%View Details
133099-07-7 -
 Darifenacin hydrobromide 99%View Details
Darifenacin hydrobromide 99%View Details
133099-07-7 -
 133099-07-7 Darifenacin hydrobromide 98%View Details
133099-07-7 Darifenacin hydrobromide 98%View Details
133099-07-7 -
 Darifenacin hydrobromide 98% (HPLC) CAS 133099-07-7View Details
Darifenacin hydrobromide 98% (HPLC) CAS 133099-07-7View Details
133099-07-7 -
 Darifenacin hydrobromide CAS 133099-07-7View Details
Darifenacin hydrobromide CAS 133099-07-7View Details
133099-07-7