DANTROLENE
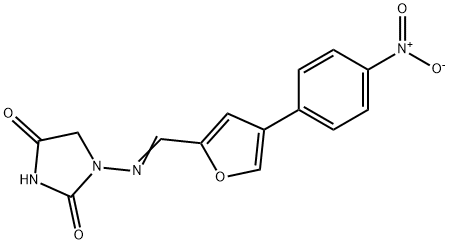
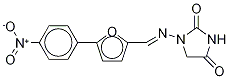
Synonym(s):1-{{[5-(4-Nitrophenyl)-2-furanyl]methylene}amino}-2,4-imidazolidinedione;Dantrolene
- CAS NO.:85008-71-5
- Empirical Formula: C14H10N4O5
- Molecular Weight: 314.25
- MDL number: MFCD00867709
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
What is DANTROLENE?
Description
Dantrolene is a drug that causes spastic muscle contraction. Unlike other muscle relaxants, it has a direct effect on the contractile mechanism by interfering in the process of calcium ion release from the sarcoplasmic reticulum. This results in a lack of coordination in the mechanism of excitation––contraction of skeletal muscle, which has a greater effect on fast muscle fibers than on slow muscle fibers.
The Uses of DANTROLENE
Dantrolene is used for controlling the onset of clinical spasticity resulting from serious clinical cases such as wounds, paralysis, cerebral palsy, and disseminated sclerosis. Synonyms of this drug are dantrium and danlen.
The Uses of DANTROLENE
Relaxant (skeletal muscle).
Biological Functions
The site of action of dantrolene is believed to be at the sarcoplasmic reticulum in skeletal muscle cells. Dantrolene binds to a calcium channel protein (ryanodine receptor) on the sarcoplasmic reticulum to close the channel and inhibit the release of calcium; the alkaloid ryanodine activates the same receptor to open the channel.
Mechanism of action
Dantrolene is believed to act directly on the contractile mechanism of skeletal muscle to decrease the force of contraction in the absence of any demonstrated effects on neural pathways, on the neuromuscular junction, or on the excitable properties of the muscle fiber membranes. Cardiac muscle and smooth muscle are minimally affected by dantrolene, likely because calcium release from sarcoplasmic reticulum of these muscle cell types occurs via a mechanism that differs from skeletal muscle. The muscle relaxant effect of dantrolene on skeletal muscle, however, is not specific, and generalized muscle weakness occurs as a major adverse side effect. Like other hydantoins, dantrolene is a weak base (pKa = 7.5) that can cross the blood-brain barrier; thus, CNS depressant side effects (e.g., sedation) are common. Dantrolene sodium salt is slowly absorbed from the gastrointestinal tract. The mean half-life of the drug in adults is approximately 9 hours after a 100-mg dose. It is slowly metabolized by the liver to give the 5-hydroxy and acetamido (nitro reduction and acetylation) metabolites, as well as unchanged drug, excreted in the urine.
Clinical Use
Dantrolene is a hydantoin derivative that acts peripherally to reduce spasticity and is indicated for use in spinal cord injury, stroke, cerebral palsy, and multiple sclerosis.
Synthesis
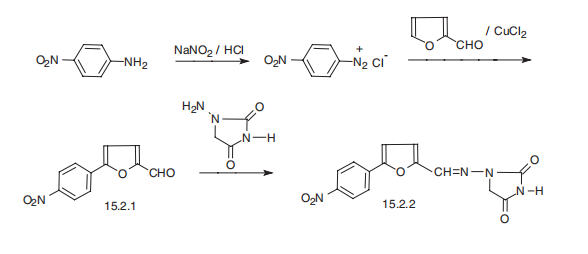
Dantrolene, 1-[[[5-(4-nitrophenyl)-2-furanyl]methylene]amino]-2,4-imidazolidinedione (15.2.2), is synthesized by reacting 4-nitrophenyldiazonium chloride with furfurol, forming 5-(4-nitrophenyl)-2-furancarboxaldehyde (15.2.1), which is reacted further with 1-aminohydantoin, to give the corresponding hydrazone, dantrolene (15.2.2).
Properties of DANTROLENE
| Melting point: | >236°C (dec.) |
| Density | 1.57±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, Refrigerator, under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Very Slightly, Sonicated) |
| form | Solid |
| pka | pKa 7.5 (Uncertain) |
| color | Yellow to Dark Yellow |
| Stability: | Hygroscopic |
Safety information for DANTROLENE
Computed Descriptors for DANTROLENE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4