Damascenone
Synonym(s):β-Damascenone;1-(2,6,6-Trimethylcyclohexa-1,3-dien-1-yl)-2-buten-1-one
- CAS NO.:23696-85-7
- Empirical Formula: C13H18O
- Molecular Weight: 190.28
- MDL number: MFCD00101024
- EINECS: 245-833-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
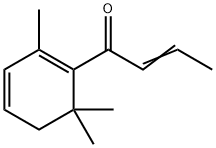
What is Damascenone?
Description
What is more evocative of Valentine’s Day than roses? But did you ever wonder about the compounds in roses that are responsible for the flower’s wonderful scent? Writing in the?Journal of Chemical Education?in 2011, Albrecht Mannschreck and Erwin von Angerer* at the University of Regensburg (Germany) gave a full accounting of roses’ aroma constituents.
According to the authors, geraniol, (–)-citronellol, and β-damascenone (Figures 1, 2, and 3, respectively) are three major components of rose scent. Geraniol is monoterpene alcohol that is a main ingredient of rose oil; it also is present in many other essential oils. It was first isolated by O. Jacobsen in 1871. Its structure is similar to that of nerol, also a rose scent ingredient.
Citronellol exists in nature as two enantiomers, designated (+) and (–). The (+)-isomer is more common and takes its name from citronella oils. The (–) isomer, however, is the one that occurs in the oils of roses and geraniums. The citronellols are also monoterpene alcohols; they are the hydrogenated forms of geraniol.
β-Damascenone is different: It belongs to a family of unsaturated ketones known as rose ketones. Its concentration in rose oil is very small, but it contributes significantly to the aroma.
All three of these compounds are articles of commerce as fragrance ingredients used by the perfume industry. So if you give your loved one perfume instead of roses for Valentine’s Day, she or he will likely get a whiff of one of them.
Occurrence
Reported found in raspberry oil, Burley tobacco, apple, lemon balm, summer savory, anise, hyssop, apple juice, cooked apple, apricot, sweet and sour cherry, black currant berries, fresh blackberry, strawberry jam, hop oil rum, white wine, red wine, black tea, tomato, rye bread, blue cheese, cow, goat and sheep milk, hop oil, beer, cognac, rum, whiskies, scotch, cider, grape wines, coffee, tea, popcorn, oatmeal, soybean, plum, prune, beans, starfruit, mango, tamarind, dill seed, corn oil, okra, cape gooseberry, anise hyssop and Roman chamomile oil.
The Uses of Damascenone
Damascenone is a fragrance ingredient that can be used in shampoos, fine fragrances, decorative cosmetics, toilet soaps as well as non-cosmetic products like household cleaners and detergents.
Preparation
By treating the corresponding ethyl safranate with allyl lithium, followed by catalytic isomerization of the reaction product; also from the oxirane derivative of β-damascone by an acid-catalyzed reaction.
Definition
ChEBI: Beta-damascenone is a cyclic monoterpene ketone that is 2,6,6-trimethylcyclohexa-1,3-diene substituted at position 1 by a crotonoyl group. It has a role as a fragrance, a volatile oil component and a plant metabolite. It is an enone, an apo carotenoid monoterpenoid and a cyclic monoterpene ketone.
Aroma threshold values
Detection: 0.0007 to 0.009 ppb. Aroma characteristics at 1.0%: sweet, brown woody, tobacco, davana-like fruity, with a spicy balsamic undernote.
Taste threshold values
Taste characteristics at 1 to 5 ppm: sweet brown, tea-like, davana dried fruity, with jamy berry, strawberry and raspberry nuances, woody, floral, herbal, green and fruity with spicy tobacco nuances.
General Description
Damascenone is a norisoprenoid ketone mainly found in red wines. It occurs naturally in tomato and grapes.
Biochem/physiol Actions
Taste at 1-5 ppm
Properties of Damascenone
| Boiling point: | 275.6±10.0 °C(Predicted) |
| Density | 0.800-0.830 g/mL at 25 °C (lit.) |
| refractive index | 1.350-1.380 |
| FEMA | 3420 | 1-(2,6,6-TRIMETHYLCYCLOHEXA-1,3-DIENYL)-2-BUTEN-1-ONE |
| Flash point: | 62°F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | liquid |
| color | yellow |
| Odor | at 10.00 % in dipropylene glycol. natural sweet fruity rose plum grape raspberry sugar |
| JECFA Number | 387 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 23696-85-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Buten-1-one, 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-(23696-85-7) |
| EPA Substance Registry System | 2-Buten-1-one, 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)- (23696-85-7) |
Safety information for Damascenone
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Damascenone
Damascenone manufacturer
Organica Aromatics Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 23696-85-7 Damascenone 99%View Details
23696-85-7 Damascenone 99%View Details
23696-85-7 -
 Damascenone CAS 23696-85-7View Details
Damascenone CAS 23696-85-7View Details
23696-85-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1