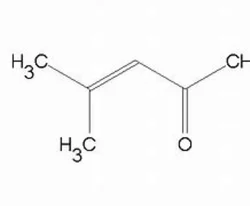
Mesityl oxide
Synonym(s):4-Methyl-3-penten-2-one;Isopropylideneacetone, 4-Methyl-3-penten-2-one;Mesityl oxide
- CAS NO.:141-79-7
- Empirical Formula: C6H10O
- Molecular Weight: 98.14
- MDL number: MFCD00008900
- EINECS: 205-502-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:31
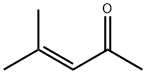
What is Mesityl oxide?
Description
The commercial grade of 4-methyl-3-penten-2-one exhibits an unpleasant odor. The pure material has a pleasant honey-like odor. This substance may be prepared by reacting acetone or diacetone alcohol with iodine or other dehydrating agent; by condensation of acetone over sulfonated polystyrene-divinylbenzene resin used as an ion exchange catalyst.
Chemical properties
Mesityl oxide is a clear, pale yellow, or colorless liquid with a strong peppermint odor. The odor threshold is 0.05 ppm.
Physical properties
Clear, pale yellow liquid with a strong, peppermint, or honey-like odor. Experimentally determined detection and recognition odor threshold concentrations were 70 μg/m3 (17 ppbv) and 200 μg/m3 (50 ppbv), respectively (Hellman and Small, 1974).
Occurrence
Reported found in orange juice, baked potato, bell pepper, sauerkraut, tomato, crisp bread, parmesan cheese, milk, chicken fat, hop oil, rum, coffee, tea, peanut, passion fruit, mushroom, tamarind, prickly pear, buckwheat, basil, elder flower, rosemary, shrimp, nectarine, clam and maté
The Uses of Mesityl oxide
Mesityl oxide is used as a solvent for resins,gums, nitrocellulose, oils, lacquers, and inks;as an insect repellant; and in ore flotation..
The Uses of Mesityl oxide
Solvent for nitrocellulose, many gums and resins, particularly vinyl resins. In lacquers, varnishes and enamels. In making methyl isobutyl ketone.
Preparation
4-Methyl-4-penten-2-one is the precursor of methyl isobutyl ketone (see Section 2.2) and can be produced from acetone in a single- or a two-step process . Pure mesityl oxide is obtained by azeotropic distillation of the crude watercontaining product. Subsequent purification by removal of the accompanying impurities, such as mesitylene and phorone [504-20-1], is effected by distillation.
Definition
ChEBI: 4-Methyl-3-penten-2-one, 9CI is an olefinic compound. It is functionally related to an acrylic acid.
Synthesis Reference(s)
Tetrahedron Letters, 27, p. 3733, 1986 DOI: 10.1016/S0040-4039(00)83866-6
General Description
Mesityl oxide appears as a colorless, oily liquid with a pungent honey-like odor. Flash point 87 °F. Less dense than water and slightly soluble in water. Vapors heavier than air. Used in paint removers, as a solvent for plastics, and as an insect repellent.
Air & Water Reactions
Highly flammable. Slightly soluble in water
Reactivity Profile
Mixing MESITYL OXIDE in equal molar proportions with any of the following substances in a closed container caused the temperature and pressure to increase: 2-aminoethanol, chlorosulfonic acid, ethylene diamine, nitric acid, oleum, or sulfuric acid [NFPA 1991].
Hazard
Flammable, moderate fire risk. Toxic by ingestion, inhalation, and skin absorption. Eye and upper respiratory tract irritant, central nervous system impairment.
Health Hazard
Inhalation causes irritation of nose and throat, headache, dizziness, difficult breathing. Contact with liquid or concentrated vapor causes severe eye irritation. Liquid irritates skin. Ingestion causes irritation of mouth and stomach.
Health Hazard
Mesityl oxide is a moderately toxic substance, more toxic than the saturated ketones.Inhalation of its vapors can cause irritationto the eyes, skin, and mucous membranes. Inhumans, the irritation effect on the eyes andnose are reported to be 25 and 50 ppm. Athigh concentrations narcosis can result. Otherthan narcosis, prolonged exposure to highconcentrations can injure the lungs, liver, and kidney. A concentration of 2500 ppm waslethal to rats.
LD50 value, oral (rats): 1120 mg/kg.
Fire Hazard
Behavior in Fire: Vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. Mildly toxic by inhalation and skin contact. Human systemic effects by inhalation: conjunctiva irritation. This compound is highly irritating to all tissues on contact; its vapors also are irritating. High concentrations are narcotic. It is readdy absorbed through intact skin. Single exposures tend to indicate that ths ketone has greater acute and narcotic action than isophorone. It can have harmful effects upon the hdneys and liver, and may damage the eyes and lungs to a serious degree. Prolonged exposure can injure liver, kidneys, and lungs. It can cause opaque cornea, keratoconus, and extensive necrosis of cornea. Dangerous fire hazard when exposed to heat, sparks, or flame; can react with oxidzing materials. Reacts violently with 2-amino ethanol, chlorosulfonic acid, ethylene diamine, HNO3, oleum, H2SO4. An insect repellent. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also KETONES.
Potential Exposure
Mesityl oxide is used as a solvent for cellulose esters and ethers and other resins in lacquers and inks. It is used in paint and varnish removers and as an insect repellent
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Storage
Color Code—Red: Flammability Hazard: Store in a flammable liquid storage area or approved cabinet away from ignition sources and corrosive and reactive materials. Mesityl oxide 1715 Prior to working with this chemical you should be trained on its proper handling and storage. Before entering confined space where this chemical may be present, check to make sure that an explosive concentration does not exist. Store in tightly closed containers in a cool, well-ventilated area away from oxidizers, strong acids. See incompatibilities above. Where possible, automatically pump liquid from drums or other storage containers to process containers.
Shipping
UN1229 Mesitly oxide, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
Purify it by distillation, preferably in a vacuum or via the semicarbazone (m 165o) which is decomposed to pure ketone. The 2,4-dinitrophenylhydrazone (m 205-206o) crystallises from EtOH. [Johnson J Am Chem Soc 73 5888 1951, Johnson J Am Chem Soc 75 2720 1953, Erskine & Waight J Chem Soc 3425 1960, Beilstein 1 H 736, 1 I 382, 1 II 793, 1 III 2995, 1 IV 3471.]
Incompatibilities
May form explosive mixture with air. May be able to form explosive peroxides. May react violently with nitric acid; aliphatic amines; alkanolamines, 2- aminoethanol, ethylene diamine; chlorosulfonic acid; oleum (fuming sulfuric acid). Not compatible with oxidizers, strong acids; strong bases; reducing agents; halogens. Dissolves some forms of plastics, resins and rubber. Attacks copper.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Mesityl oxide
| Melting point: | −53 °C(lit.) |
| Boiling point: | 129 °C(lit.) |
| Density | 0.858 g/mL at 25 °C(lit.) |
| vapor pressure | 10.5 hPa (20 °C) |
| refractive index | n |
| FEMA | 3368 | 4-METHYL-3-PENTEN-2-ONE |
| Flash point: | 87 °F |
| storage temp. | Store below +30°C. |
| solubility | 30g/l |
| form | Liquid, Free Of Suspended Matter |
| color | Clear |
| Specific Gravity | 0.86 |
| Odor | Strong; peppermint; honeylike. |
| explosive limit | 1.4-10.1%(V) |
| Water Solubility | 28 G/L (20 ºC) |
| Merck | 14,5908 |
| JECFA Number | 1131 |
| BRN | 1361550 |
| Henry's Law Constant | (x 10-6 atm?m3/mol):
4.01 at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| Dielectric constant | 15.4(20℃) |
| Exposure limits | TLV-TWA 60 mg/m3 (15 ppm) (ACGIH),
10-h TWA 40 mg/m3 (10 ppm) (NIOSH);
STEL 100 mg/m3 (25 ppm); IDLH 5000
ppm. |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 141-79-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 3-Penten-2-one, 4-methyl-(141-79-7) |
| EPA Substance Registry System | Mesityl oxide (141-79-7) |
Safety information for Mesityl oxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Mesityl oxide
Mesityl oxide manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 141-79-7 98%View Details
141-79-7 98%View Details
141-79-7 -
 Mesityl Oxide CAS 141-79-7View Details
Mesityl Oxide CAS 141-79-7View Details
141-79-7 -
 Mesityl oxide CAS 141-79-7View Details
Mesityl oxide CAS 141-79-7View Details
141-79-7 -
 Mesityl oxide, puriss 99%+ CAS 141-79-7View Details
Mesityl oxide, puriss 99%+ CAS 141-79-7View Details
141-79-7 -
 MESITYL OXIDE 90%View Details
MESITYL OXIDE 90%View Details
141-79-7 -
 MESITYL OXIDE (141-79-7 )View Details
MESITYL OXIDE (141-79-7 )View Details
141-79-7 -
 Mesityl oxideView Details
Mesityl oxideView Details
141-79-7 -
 Mesityl Oxide ChemicalView Details
Mesityl Oxide ChemicalView Details
141-79-7
