Methyl vinyl ketone
Synonym(s):Methyl vinyl ketone;Vinyl methyl ketone
- CAS NO.:78-94-4
- Empirical Formula: C4H6O
- Molecular Weight: 70.09
- MDL number: MFCD00008777
- EINECS: 201-160-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
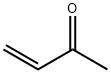
What is Methyl vinyl ketone?
Description
Methyl vinyl ketone (MVK) is a stable, highly flammable, heatand light-sensitive, and colourless liquid. It is incompatible with strong bases, strong oxidising agents, and strong reducing agents and undergoes autopolymerisation. MVK is a reactive organic compound classified as an enone. It is a colourless to yellow, flammable, highly toxic liquid with a pungent odour. It is easily soluble in water, methanol, ethanol, and acetic acid. It can act as an alkylating agent. Its alkylating ability is both the source of its high toxicity and the feature that makes it a useful intermediate in organic synthesis. It polymerises spontaneously and is used in the manufacture of plastic polymers. It is also an intermediate in the synthesis of steroids and vitamin A.
Chemical properties
3-Buten-2-one is a colorless liquid with a penetrating odor. The compound is soluble in water and in organic solvents. Pure 3-buten-2-one is stable only below 0 ℃; it polymerizes rapidly at room temperature. It is stabilized for storage and transportation by the addition of hydroquinone and glacial acetic acid (0.1 wt % each).
The Uses of Methyl vinyl ketone
Alkylating agent; commercial starting material for plastics; as intermediate in the synthesis of steroids and vitamin A.
Definition
ChEBI: Buten-2-one is a methyl ketone that is butan-2-one with an unsaturation at position 3. It is a methyl ketone and an enone. It is functionally related to a butan-2-one and a but-1-ene.
General Description
A clear colorless liquid with a pungent odor. Flash point 20 °F. May polymerize with the release of heat under exposure to heat or contamination. Less dense than water. Highly toxic by inhalation. Causes burns to skin, eyes and mucous membranes.
Air & Water Reactions
Highly flammable. Miscible with water. Unstable in the presence of heat, light and air.
Reactivity Profile
Methyl vinyl ketone is incompatible with strong oxidizers and strong bases. Methyl vinyl ketone polymerizes spontaneously upon exposure to heat or sunlight. This polymerization may cause violent ruptures in containers. .
Health Hazard
Methyl vinyl ketone is readily absorbed through the skin, causing general poisoning, similar to other ketones; inhalation has central nervous system depressant effects. It is irritating to mucous membranes and respiratory tract and to the skin; it is a lachrymator and can cause eye injury.
Fire Hazard
Vapors form flammable mixtures with air, and may travel a considerable distance to a source of ignition and flash back. Polymerization may take place in containers, possibly with violent rupture of containers. Upon exposure to heat or flame, Methyl vinyl ketone emits toxic and irritating fumes. Container may explode in heat of fire. Vapor explosion and poison hazard indoors, outdoors, or in sewers. Polymerizes on standing. Hazardous polymerization may occur. Avoid heat or sunlight.
Safety Profile
Poison by ingestion, inhalation, and intraperitoneal routes. A severe irritant to skin, eyes, and mucous membranes. A lachrymator. Mutation data reported. See also KETONES. Dangerous fire hazard when exposed to heat, flame, or oxidizers. To fight fire, use CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes.
Potential Exposure
Methyl vinyl ketone is used as an alkylating agent, a starting material for plastics; and an intermediate in the synthesis of steroids and Vitamin A.
First aid
inhalation has central nervous system depressant effects. Itis irritating to mucous membranes and respiratory tract andto the skin; it is a lachrymator and can cause eye injury.Liquid or high concentration of vapors causes blistering ofthe skin. Similar to other ketones, it can cause sore throat,sneezing, coughing, and salivation. Inhalation may causenausea and vomiting; inhalation of high concentrations cancause headache, dizziness, fainting, tremor, uncoordination,lowered body temperature, depressed respiratory and heartrate, gasping, coma, and death. Direct aspiration of liquidinto lungs can cause chemical pneumonia.
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Before entering confined space where this chemicalmay be present, check to make sure that an explosive concentration does not exist. Protect against physical damage.Outside or detached storage is preferred. Inside storageshould be in a standard flammable liquids storage room.Separate from oxidizing materials. MVK vapors are uninhibited and may form polymers in the flame arresters ofstorage tanks, resulting in stoppage of vent.
Shipping
UN1251 Methyl vinyl ketone, stabilized, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, 8-Corrosive material, Inhalation Zone A.
Purification Methods
It forms an 85% azeotrope with water. After drying with K2CO3 and CaCl2 (with cooling), the ketone is distilled at low pressures. [Beilstein 1 IV 3444.]
Incompatibilities
Vapors may form explosive mixture with air. Heat or shock may cause explosive polymerization. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrated amines, azo, diazo, azido compounds, carbamates, organic cyanates.
Properties of Methyl vinyl ketone
| Melting point: | -7°C |
| Boiling point: | 80 °C |
| Density | 0.864 g/mL at 25 °C(lit.) |
| vapor density | 1.3 (vs air) |
| vapor pressure | 310 mm Hg ( 55 °C) |
| refractive index | n |
| Flash point: | 20 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Sparingly), Methanol (Slightly) |
| form | Liquid |
| color | Clear colorless to pale yellow, may discolor to brown on storage |
| Specific Gravity | 0.864 |
| Odor | at 100.00?%. sweet |
| explosive limit | 15.64% |
| Water Solubility | MISCIBLE |
| Merck | 14,6134 |
| BRN | 506021 |
| Stability: | Stable. Incompatible with strong bases, strong oxidizing agents, strong reducing agents. Heat and light sensitive. May undergo autopolymerization. Highly flammable. Refrigerate. |
| CAS DataBase Reference | 78-94-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Methyl vinyl ketone(78-94-4) |
| EPA Substance Registry System | Methyl vinyl ketone (78-94-4) |
Safety information for Methyl vinyl ketone
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H314:Skin corrosion/irritation H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methyl vinyl ketone
Methyl vinyl ketone manufacturer
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Methyl vinyl ketone, 90%, stab. with 0.5% hydroquinone CAS 78-94-4View Details
Methyl vinyl ketone, 90%, stab. with 0.5% hydroquinone CAS 78-94-4View Details
78-94-4 -
 Methyl vinyl ketone CAS 78-94-4View Details
Methyl vinyl ketone CAS 78-94-4View Details
78-94-4 -
 Methyl vinyl ketone, 95%, stab. with 0.5% hydroquinone CAS 78-94-4View Details
Methyl vinyl ketone, 95%, stab. with 0.5% hydroquinone CAS 78-94-4View Details
78-94-4 -
 Methyl vinyl ketone CAS 78-94-4View Details
Methyl vinyl ketone CAS 78-94-4View Details
78-94-4 -
 METHYL VINYL KETONE CAS: 78-94-4View Details
METHYL VINYL KETONE CAS: 78-94-4View Details
78-94-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
