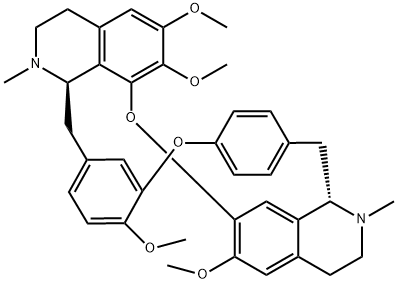
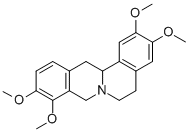
D-Tetrandrine
Synonym(s):(1β)-6,6′,7,12-Tetramethoxy-2,2′-dimethylberbaman
- CAS NO.:518-34-3
- Empirical Formula: C38H42N2O6
- Molecular Weight: 622.75
- MDL number: MFCD00082551
- EINECS: 683-095-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is D-Tetrandrine?
Description
Recently it has been discovered that pronounced drug-dependence and related toxic effects occur in both dogs and rhesus monkeys with this alkaloid on intravenous injection with a dose of 10-150 mg/kg. When rapidly injected, the acute hypotensive effect is very marked and fatal at once. Following drug administration at toxic levels it is found that severe local tissue reaction heptaotoxicity and lymphoid necrosis occurs. At the highest dosage level there is a very definite nephrotoxicity in monkeys and some indications of this in dogs. The evidence available suggests that monkeys are less sensitive to hepatotoxicity with this drug than dogs.
Description
Tetrandrine is an alkaloid that was isolated from the root of the Asian herb Stephania tetrandra by H. Kando and K. Yano in 1932. It is a constituent of the Chinese herbal drug han-fang-chi that is used as an analgesic and antipyretic.
Tetrandrine is a calcium channel blocker that is currently being developed as an Ebola drug. R. A. Davey at the Texas Biomedical Research Institute (San Antonio) suspected that calcium signaling is crucial to the ebolavirus infection process. He and researchers around the world showed that two-pore calcium channel 2 is the key signaling pathway. Tetrandrine may be more effective as a prophylactic treatment than as a drug for the active disease, but it has many hurdles to overcome before it is approved for commercial use.
Description
Tetrandrine is a bis-benzylisoquinoline alkaloid that has been found in R. stephania roots and has diverse biological activities. It induces autophagy in HeLa, MCF-7, and human foreskin fibroblast (HFF) cells when used at a concentration of 5 μM, an effect that can be reversed by the autophagy inhibitor 3-methyladenine . Tetrandrine inhibits PAF-, thrombin-, collagen-, ADP-, or epinephrine-induced aggregation of isolated human platelets. Priming of mesenchymal stem cells (MSCs) with tetrandrine (5 and 10 μM) reduces TNF-α secretion by RAW 264.7 cells in co-culture. Ear skin transplantation of tetrandrine-primed MSCs decreases ear levels of TNF-α in a mouse model of ear skin inflammation. Tetrandrine (1 mg/kg) increases soleus muscle levels of glucose transporter 4 (Glut4) and decreases plasma glucose levels in a rat model of diabetes induced by streptozotocin .
Chemical properties
White powder
Physical properties
Appearance: Needle-like crystals (ether). Solubility: Hardly soluble in water and petroleum ether; soluble in ether and some organic solvents. Melting point: 219– 222?°C. Specific optical rotation: 285° (c?=?1, CHCl3); sensitive to light.
History
Recent studies have shown that tetrandrine has a variety of biological effects and
very good applicational prospects in the treatment of fibrosis and portal vein and
pulmonary hypertension, the regulation of immunologic function, as well as the
prevention and treatment of tumor.
As early as 1988, tetrandrine has been found having the effect on blocking the
Ca2 + channel and was quickly applied into the pharmacological research in the field
of cardiovascular and inflammatory diseases. Results from a large number of
studies have shown that tetrandrine has good effects on antihypertension, arrhythmia, myocardial ischemia, inflammation, and so on. As a traditional Chinese
calcium antagonist, tetrandrine has a broad prospects in clinical applications of cardiovascular and inflammatory diseases.In the early 1990s, the application of tetrandrine was extended. During that time,
researchers conducted many studies about its protective effects on liver, lung, and
mitochondria, which opened a new field for the treatment of liver disease. In
2002, it was found that tetrandrine can inhibit the synthesis of DNA and RNA in
tumor cells, which provided a new method for the treatment of cancer.
At present, the prevention and treatment effects of hypertension, fibrosis, digestive diseases, tumors, rheumatoid arthritis, and other autoimmune diseases of tetrandrine have been confirmed, as well as the function of reducing portal hypertension
and pulmonary hypertension, while its other pharmacological effects are to be
explored in further study.
The Uses of D-Tetrandrine
analgesic, antineoplastic, antihypertensive, lymphotoxin
The Uses of D-Tetrandrine
Tetrandrine is a lipopolysaccharide-induced microglial suppressor, effectively reducing the production of bacterial inflammatory mediators. Anti-inflammatory, anti-nociceptive. Tetrandrine is used in China to treat high blood pressure. This drug blocked the TPC2 calcium channel required for the Ebola infection process (Robert A. Davey et al., Science 2015, DOI: 10.1126/science.1258758).
What are the applications of Application
Tetrandrine is a calcium channel protein inhibitor
Indications
This product is included in national standards for chemical drugs (Volume 14), British Pharmacopoeia (2017), and European Pharmacopoeia (9.0th ed.). Tetrandrine is used for the treatment of mild to moderate hypertension and hypertensive crisis, rheumatism, silicosis, etc.
Definition
ChEBI: (+)-Tetrandrine is a member of isoquinolines and a bisbenzylisoquinoline alkaloid.
Pharmacology
Tetrandrine has analgesic, anti-inflammatory, and anti-allergic effects and has a wide range of usage on the cardiovascular system owing to its antihypertensive, anti-myocardial ischemia/reperfusion injury and antiarrhythmic effects. It can inhibit the platelet aggregation induced by ADP, collagen, and arachidonic acid in?vitro and can also restrain the platelet adhesion and thrombosis (in rabbits). Tetrandrine also has anticancer effects. Studies have shown that tetrandrine has a strong inhibitory effect on the DNA and RNA synthesis in L7712 and S180 (cancer cells), which can significantly suppress the growth of Wacker sarcoma W256. Besides that, tetrandrine has the ability to relax the striated muscle, and its methyl iodide or methyl bromide derivatives can also affect the muscles. Notably, tetrandrine can prevent silicosis and has a preferable outcome on the clinical treatment of such disease. In addition, tetrandrine also owns antipyretic, diuretic, and antiallergic shock effects.
Clinical Use
Tetrandrine is used for the treatment of hypertension, angina, termination of paroxysmal supraventricular tachycardia, pulmonary fibrosis, and other diseases in clinical application, and it also has strong antitumor effects. Tetrandrine was also approved for lowering blood glucose and free radical damage; its treatment effect on silicosis is significant and superior to conventional immunosuppressive and cytotoxic drugs.
References
Gralla, Coleman, Jonas, Cancer Chemother. Rep., Pt. 3, 5(1), 79 (1974)
Properties of D-Tetrandrine
| Melting point: | 219-222 °C(lit.) |
| alpha | 285 º (c=1, CHCl3) |
| Boiling point: | 662.81°C (rough estimate) |
| Density | 1.1528 (rough estimate) |
| refractive index | 1.5300 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly, Heated) |
| form | solid |
| pka | 7.70±0.20(Predicted) |
| color | Off-White |
| λmax | 283nm(EtOH)(lit.) |
| Merck | 14,9231 |
Safety information for D-Tetrandrine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for D-Tetrandrine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
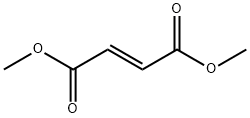


You may like
-
 Tetrandrine CAS 518-34-3View Details
Tetrandrine CAS 518-34-3View Details
518-34-3 -
 Tetrandrine 99% (HPLC) CAS 518-34-3View Details
Tetrandrine 99% (HPLC) CAS 518-34-3View Details
518-34-3 -
 Tetrandrine CAS 518-34-3View Details
Tetrandrine CAS 518-34-3View Details
518-34-3 -
 Tetrandrine CAS 518-34-3View Details
Tetrandrine CAS 518-34-3View Details
518-34-3 -
 Tetrandrine CAS 518-34-3View Details
Tetrandrine CAS 518-34-3View Details
518-34-3 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
