D(+)-Malic acid
Synonym(s):(R)-(+)-2-Hydroxysuccinic acid;(R)-(+)-Malic acid;D- (+)-Malic acid;D -Hydroxybutanedioic acid;D-(+)-Malic acid
- CAS NO.:636-61-3
- Empirical Formula: C4H6O5
- Molecular Weight: 134.09
- MDL number: MFCD00004245
- EINECS: 211-262-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
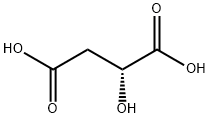
What is D(+)-Malic acid?
Chemical properties
white to light yellow crystal powde
The Uses of D(+)-Malic acid
The naturally occuring isomer is the L-form which has been found in apples and many other fruits and plants. Selective α-amino protecting reagent for amino acid derivatives. Versatile synthon for the preparation of chiral compounds including κ-opioid rece
The Uses of D(+)-Malic acid
D-(+)-Malic acid used as an acidulant and flavoring agent, food additive. And it is also used in the place of the less sour citric acid in sour sweets.
The Uses of D(+)-Malic acid
D-(+)-Malic acid can be used:
- As a starting material for the enantioselective total synthesis of (?)-erinapyrone B.
- As a chiral organocatalyst in the synthesis of α-aminophosphonates from various aldehydes, aniline, and diethyl phosphite.
Definition
ChEBI: An optically active form of malic acid having (R)-configuration.
Biological Activity
D-(+)-Malic acid, an active isomer of Malic acid, is a competitive inhibitor of L(--)malic acid transport.
Synthesis
Malic acid is isolated from immature apples; industrially prepared is obtained by catalytic oxidation of benzene, then reacting with water at high temperature and high pressure to generate maleic anhydride.
in vitro
Some bacteria belonging to Arthrobacter, Brevibacterium, Corynebacterium, Pseudomonas, Bacillus, and Acinetobacter produced D-(+)-Malic acid (D-Malic acid) from Maleic acid when the cells grown in a medium containing citraconic acid are reacted aerobically with Maleic acid in the pH 7.0 phosphate buffer containing 0.1% sodium chloride.
Properties of D(+)-Malic acid
| Melting point: | 98-102 °C (lit.) |
| Boiling point: | 167.16°C (rough estimate) |
| alpha | 2.2 º (c=3, H2O) |
| Density | 1.60 |
| refractive index | 6.5 ° (C=10, Acetone) |
| storage temp. | Store below +30°C. |
| solubility | Soluble in methanol, ethanol, acetone, ether. |
| form | Crystalline Powder |
| pka | 3.61±0.23(Predicted) |
| color | White |
| PH | 2.2 (10g/l, H2O, 20℃) |
| optical activity | [α]20/D +28.0±2°, c = 5.5% in pyridine |
| Water Solubility | soluble |
| Merck | 14,5707 |
| BRN | 1723540 |
| CAS DataBase Reference | 636-61-3(CAS DataBase Reference) |
| NIST Chemistry Reference | (r)-Hydroxybutanedioic acid(636-61-3) |
Safety information for D(+)-Malic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for D(+)-Malic acid
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 D-(+)-Malic Acid CAS 636-61-3View Details
D-(+)-Malic Acid CAS 636-61-3View Details
636-61-3 -
 D-(+)-Malic acid, ≥99% CAS 636-61-3View Details
D-(+)-Malic acid, ≥99% CAS 636-61-3View Details
636-61-3 -
 D-(+)-Malic acid 98% (HPLC) CAS 636-61-3View Details
D-(+)-Malic acid 98% (HPLC) CAS 636-61-3View Details
636-61-3 -
 D-( )-Malic acid 97% CAS 636-61-3View Details
D-( )-Malic acid 97% CAS 636-61-3View Details
636-61-3 -
 D-(+)-Malic acid CAS 636-61-3View Details
D-(+)-Malic acid CAS 636-61-3View Details
636-61-3 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
